The Role and Importance of Clinical Research Coordinators
Clinical research coordinators (CRCs) are crucial in the healthcare and pharmaceutical industries. They manage clinical trials, ensuring they adhere to regulatory requirements and scientific standards. Their role involves coordinating between different stakeholders, including patients, healthcare providers, and researchers. This blog delves into the responsibilities, qualifications, and career prospects of CRCs, with a particular focus on their salaries, especially at renowned institutions like Massachusetts General Hospital.
Responsibilities of Clinical Research Coordinators
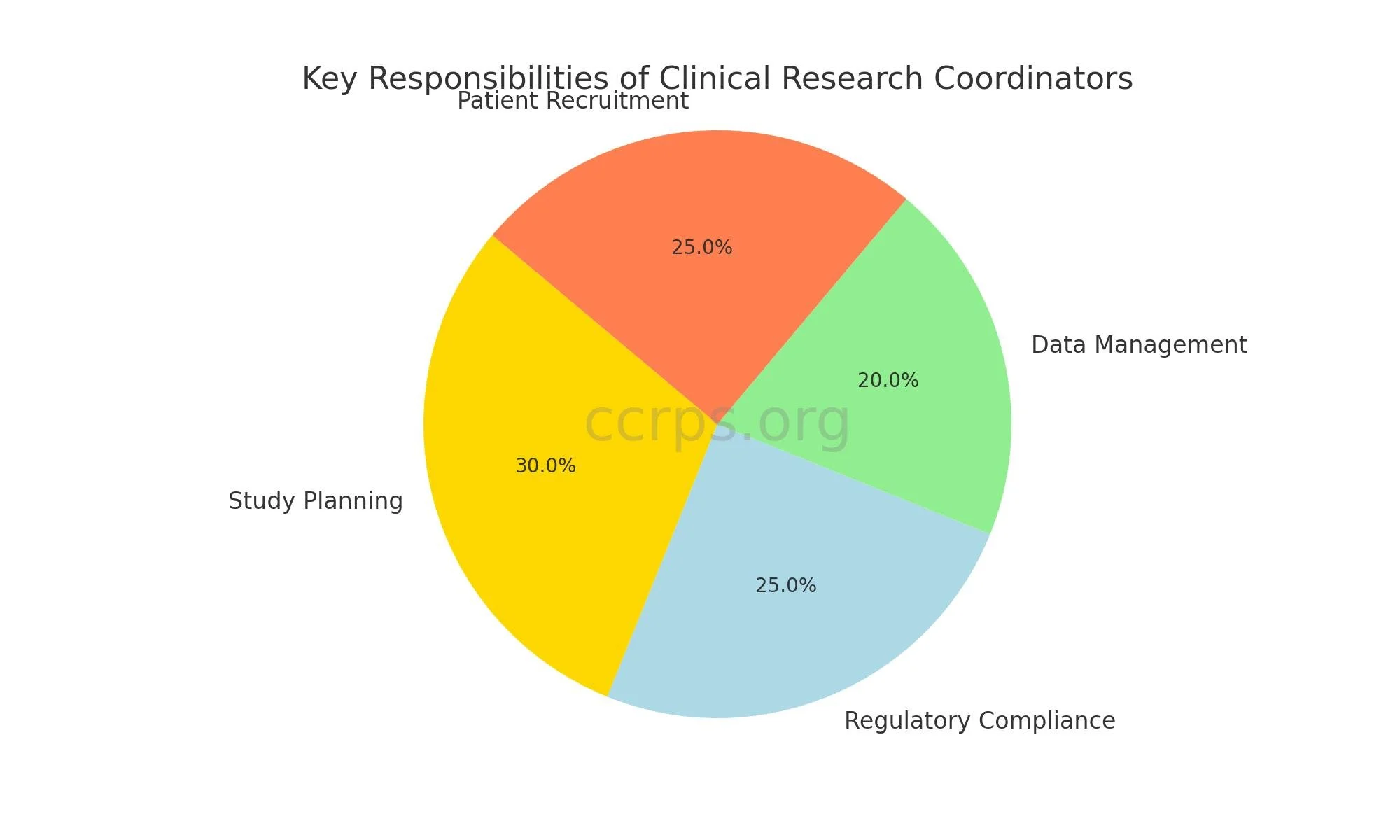
Clinical research coordinators perform various tasks to ensure clinical trials' smooth and efficient conduct. Their responsibilities include:
Study Planning and Coordination: CRCs assist in developing study protocols, managing study timelines, and coordinating various trial phases.
Regulatory Compliance: They ensure all trials comply with regulatory requirements, including obtaining necessary approvals from Institutional Review Boards (IRBs).
Data Management: CRCs are responsible for data collection, entry, and maintenance, ensuring data integrity and confidentiality.
Patient Recruitment and Communication: They recruit and screen potential study participants, provide informed consent, and maintain ongoing communication with participants throughout the study.
References
Qualifications and Skills Required
To become a clinical research coordinator, certain educational and professional qualifications are typically required:
Educational Background: A bachelor's degree in life sciences, nursing, or a related field is usually required. Advanced degrees or certifications can be advantageous.
Certifications: Professional certifications such as the Certified Clinical Research Coordinator (CCRC) can enhance job prospects and credibility.
Skills: Essential skills include strong organizational abilities, attention to detail, excellent communication skills, and proficiency in using clinical trial management software (CTMS).
References
Career Prospects and Salary
The career prospects for clinical research coordinators are promising, with a growing demand for clinical trials. Salaries can vary widely based on experience, education, and location. For example, clinical research coordinators at Massachusetts General Hospital enjoy competitive salaries due to the institution's prestigious reputation.
Salary Insights at Massachusetts General Hospital
At Massachusetts General Hospital, clinical research coordinators can expect to earn salaries that reflect their expertise and the institution's standards. According to recent data, the average salary for a CRC at Massachusetts General Hospital is around $60,000 per year, with opportunities for growth based on experience and additional qualifications.
References
The Impact of Clinical Research Coordinators
Clinical research coordinators play a pivotal role in advancing medical research and improving patient care. Their contributions ensure the successful implementation of clinical trials, leading to the development of new treatments and therapies.
Key Contributions
Advancing Medical Knowledge: By managing clinical trials, CRCs contribute to the generation of new medical knowledge and innovations.
Ensuring Patient Safety: They ensure that trials are conducted safely and ethically, prioritizing patient welfare.
Facilitating Regulatory Approval: CRCs help navigate the complex regulatory landscape, facilitating the approval of new drugs and treatments.
References
Challenges Faced by Clinical Research Coordinators
Despite the rewarding nature of their work, clinical research coordinators face several challenges:
Regulatory Hurdles: Navigating complex regulatory requirements can be challenging and time-consuming.
Participant Recruitment: Recruiting and retaining study participants can be difficult, impacting study timelines.
Data Management: Ensuring the accuracy and confidentiality of clinical trial data is crucial and demanding.
References
Enhancing the Role of Clinical Research Coordinators
To address these challenges and enhance the role of CRCs, several strategies can be implemented:
Training and Education: Continuous training and education programs can help CRCs stay updated with regulatory changes and new methodologies.
Technology Integration: Utilizing advanced clinical trial management systems can streamline data management and improve efficiency.
Collaboration and Support: Encouraging collaboration among stakeholders and providing adequate support can alleviate some of the challenges CRCs face.
References
Future Trends in Clinical Research Coordination
The field of clinical research coordination is evolving, with several trends shaping its future:
Increased Use of Technology: Technology, including artificial intelligence and machine learning, is increasingly being integrated into clinical trials to enhance efficiency and accuracy.
Focus on Patient-Centered Trials: There is a growing emphasis on patient-centered approaches, ensuring that trials are designed with the patient experience in mind.
Global Collaboration: International collaboration is becoming more common, enabling more extensive and diverse clinical trials.
References
Conclusion
Clinical research coordinators are integral to the success of clinical trials, playing a vital role in advancing medical research and improving patient care. Their contributions, challenges, and future prospects highlight the importance of this profession. As the field continues to evolve, CRCs will remain at the forefront of medical innovation, ensuring the safe and effective conduct of clinical trials.
The Role of Physician Assistants in Clinical Research
Role of Physician Assistants in Clinical Research
The healthcare industry is a dynamic field that continuously evolves with new research and advancements. Physician assistants (PAs) play a vital role in this landscape, not only in clinical settings but also in research. This blog delves into the involvement of physician assistants in clinical research, shedding light on their contributions, responsibilities, and the impact of their work on medical advancements.
Understanding the Role of Physician Assistants
Physician assistants are licensed medical professionals who practice under the supervision of physicians. They are trained to perform a wide range of medical tasks, from diagnosing and treating illnesses to prescribing medications. The scope of their practice can vary depending on the state or country regulations, but they generally work in various healthcare settings, including hospitals, clinics, and private practices.
Key Responsibilities of Physician Assistants
Conducting patient examinations
Diagnosing and treating illnesses
Performing medical procedures
Prescribing medications
Educating patients about preventive care
Assisting in surgeries
Collaborating with healthcare teams
Physician Assistants in Clinical Research
Clinical research is a crucial aspect of the medical field, leading to new treatments, medications, and improved patient care practices. Physician assistants are increasingly involved in clinical research, contributing their medical expertise and patient care skills to advance medical knowledge.
Involvement in Research Studies
Physician assistants can participate in various types of clinical research studies, including:
Clinical Trials: PAs can help design, conduct, and monitor clinical trials, ensuring they adhere to ethical standards and regulatory requirements.
Observational Studies: In these studies, PAs collect and analyze data from patient observations to identify trends and outcomes.
Translational Research: PAs bridge the gap between laboratory research and clinical applications, helping translate scientific discoveries into practical treatments.
Responsibilities in Research Settings
The specific roles of PAs in clinical research can vary, but their responsibilities often include:
Patient Recruitment: Identifying and enrolling eligible patients for research studies.
Data Collection: Gathering accurate and comprehensive data from patient interactions, medical records, and laboratory results.
Patient Monitoring: Ensuring patient safety and adherence to study protocols throughout the research process.
Data Analysis: Assisting in the interpretation of research data to draw meaningful conclusions.
Ethical Compliance: Ensuring that research studies comply with ethical guidelines and regulatory standards.
Benefits of PA Involvement in Research
The involvement of physician assistants in clinical research offers several benefits:
Improved Patient Care: PAs bring a patient-centered approach to research, focusing on the well-being and safety of participants.
Enhanced Data Accuracy: Their clinical expertise ensures accurate data collection and interpretation.
Increased Efficiency: PAs can streamline research processes, reducing the time and resources needed to conduct studies.
Broader Perspectives: The diverse backgrounds of PAs contribute to a more comprehensive understanding of research outcomes.
Challenges and Opportunities
While physician assistants have much to offer in clinical research, they also face certain challenges. Understanding these challenges and opportunities can help optimize their involvement in research settings.
Challenges
Time Constraints: Balancing clinical duties with research responsibilities can be demanding.
Training Requirements: Additional training in research methodologies and regulatory compliance may be necessary.
Limited Recognition: The contributions of PAs in research may not always be fully recognized or appreciated.
Opportunities
Professional Development: Engaging in research provides PAs with opportunities for professional growth and development.
Collaboration: Research settings offer a platform for PAs to collaborate with other healthcare professionals and researchers.
Impact on Healthcare: PAs can make significant contributions to medical advancements, improving patient outcomes and healthcare practices.
Case Studies and Examples
To illustrate the impact of physician assistants in clinical research, let's explore some real-world examples and case studies.
Case Study 1: PA-Led Clinical Trial
In a clinical trial investigating a new treatment for diabetes, a team of physician assistants played a crucial role. They were involved in patient recruitment, data collection, and patient monitoring. Their efforts ensured that the trial was conducted smoothly and ethically, leading to the successful development of a new medication that improved the lives of many patients.
Case Study 2: Observational Study on Hypertension
A group of PAs conducted an observational study to understand the long-term effects of hypertension in elderly patients. By collecting and analyzing patient data, they identified key factors contributing to improved blood pressure management. Their findings were published in a leading medical journal, influencing hypertension treatment guidelines.
Skills and Qualifications for Research-Involved PAs
Physician assistants who wish to engage in clinical research should possess specific skills and qualifications to excel in this field.
Essential Skills
Clinical Expertise: A strong foundation in clinical practice is essential for understanding research contexts and patient care.
Analytical Skills: Ability to analyze data, identify trends, and draw meaningful conclusions.
Attention to Detail: Precision in data collection and documentation to ensure accuracy and reliability.
Communication Skills: Effective communication with research teams, patients, and regulatory bodies.
Recommended Qualifications
Research Training: Additional training or certification in clinical research methodologies and regulatory compliance.
Advanced Degrees: Pursuing advanced degrees (e.g., Master's or PhD) in related fields can enhance research capabilities.
Experience: Gaining experience through participation in research projects or clinical trials.
Professional Development Opportunities
Physician assistants interested in clinical research can pursue various professional development opportunities:
Workshops and Seminars: Attending workshops and seminars on clinical research topics.
Certifications: Obtaining certifications from recognized institutions (e.g., Clinical Research Certification from the Association of Clinical Research Professionals).
Networking: Joining professional organizations and networks focused on clinical research.
Future Trends and Developments
The role of physician assistants in clinical research is expected to grow as the healthcare industry continues to evolve. Emerging trends and developments will shape their involvement in research settings.
Technological Advancements
Digital Health Tools: The use of digital health tools and technologies will enhance data collection, analysis, and patient monitoring.
Telemedicine: Telemedicine will enable PAs to conduct research remotely, expanding their reach and impact.
Artificial Intelligence: AI and machine learning will assist PAs in data analysis and predictive modeling.
Collaborative Research
Interdisciplinary Teams: PAs will collaborate with interdisciplinary teams, including physicians, nurses, and researchers, to conduct comprehensive studies.
Global Research Networks: Participation in global research networks will provide PAs with access to diverse patient populations and resources.
Policy and Regulatory Changes
Expanded Roles: Policy changes may expand the roles of PAs in research, recognizing their contributions and providing more opportunities.
Ethical Standards: Ongoing updates to ethical standards and regulatory guidelines will ensure the integrity and quality of clinical research.
Finalization
Physician assistants play a crucial role in clinical research, contributing their clinical expertise, patient care skills, and dedication to advancing medical knowledge. Their involvement in research offers numerous benefits, including improved patient care, accurate data collection, and efficient research processes. As the healthcare industry continues to evolve, the role of PAs in clinical research will become increasingly significant, shaping the future of medical advancements and patient outcomes.
References
American Academy of Physician Assistants. (2023). The Role of PAs in Clinical Research.
Clinical Research Society. (2022). Physician Assistants and Clinical Trials.
Journal of Clinical Research. (2021). Observational Studies Conducted by PAs.
National Institutes of Health. (2023). PA Contributions to Translational Research.
Association of Clinical Research Professionals. (2022). Certification Programs for PAs in Research.
Health Research Policy. (2022). Impact of PA-Led Research Studies.
Digital Health Journal. (2023). Technological Advancements in Clinical Research.
Global Research Network. (2021). Collaborative Research Opportunities for PAs.
Clinical Research Ethics. (2023). Ethical Standards in Clinical Research.
Medical Research Review. (2022). Future Trends in Clinical Research.
The Comprehensive Guide to Medical Research Consultant Jobs
Medical Research Consultant Jobs
Medical research consultants play a crucial role in the healthcare industry, bridging the gap between research and practical application. They provide expert advice, conduct studies, and help shape healthcare policies and practices. This blog explores the essential aspects of medical research consultant jobs, including the required skills, typical responsibilities, career paths, and the current job market trends.
Understanding the Role of a Medical Research Consultant
A medical research consultant is a professional who offers specialized knowledge and expertise in medical research to organizations such as pharmaceutical companies, hospitals, universities, and government agencies. These consultants are instrumental in designing, conducting, and analyzing clinical trials, ensuring that research findings are valid and applicable.
Key Responsibilities
Medical research consultants have a wide range of responsibilities, including:
Designing Research Studies: Developing research protocols and methodologies to ensure that studies are scientifically sound.
Conducting Research: Overseeing the implementation of research studies, including data collection and analysis.
Consulting with Clients: Providing expert advice to clients on research-related issues, including study design, regulatory compliance, and data interpretation.
Preparing Reports and Publications: Writing detailed reports and scientific papers based on research findings.
Regulatory Compliance: Ensuring that all research activities comply with relevant regulations and ethical standards.
Required Skills and Qualifications
To excel as a medical research consultant, one must possess a combination of educational qualifications, technical skills, and soft skills.
Educational Background: A minimum of a master's degree in a relevant field such as medical sciences, public health, or clinical research is typically required. A Ph.D. is often preferred.
Technical Skills: Proficiency in statistical analysis, research methodologies, and data interpretation. Familiarity with software such as SPSS, SAS, and R is advantageous.
Soft Skills: Strong communication, problem-solving, and project management skills. The ability to work collaboratively with diverse teams is essential.
Career Path and Advancement
The career path for a medical research consultant often begins with roles such as research assistant or clinical research coordinator. With experience, professionals can advance to senior consultant positions or take on leadership roles in research organizations.
Career Progression:
Entry-Level Positions: Research Assistant, Clinical Research Coordinator
Mid-Level Positions: Research Analyst, Clinical Research Associate
Senior-Level Positions: Senior Consultant, Principal Investigator, Research Director
The Job Market for Medical Research Consultants
The demand for medical research consultants is influenced by various factors, including advancements in medical technology, the increasing complexity of clinical trials, and the growing emphasis on evidence-based medicine.
Industry Demand
Several industries actively seek medical research consultants, including:
Pharmaceutical Companies: To develop and test new drugs.
Healthcare Providers: To improve patient care and treatment protocols.
Government Agencies: To shape public health policies and regulatory frameworks.
Academic Institutions: To conduct cutting-edge research and mentor students.
Job Outlook
The job outlook for medical research consultants is positive, with the Bureau of Labor Statistics projecting steady growth in related fields. This growth is driven by the continuous need for medical research to address emerging health challenges and improve healthcare outcomes.
Essential Tips for Aspiring Medical Research Consultants
For those aspiring to become medical research consultants, here are some essential tips to help you succeed in this competitive field:
Pursue Relevant Education and Training
Obtain a degree in medical sciences, public health, or a related field.
Consider advanced degrees or certifications, such as the Advanced Clinical Medical Scribe Certification Course.
Participate in internships and research projects to gain practical experience.
Build a Strong Professional Network
Join professional organizations, such as the Association of Clinical Research Professionals (ACRP) and the Society for Clinical Research Sites (SCRS).
Attend conferences, workshops, and seminars to stay updated on industry trends and connect with peers.
Develop Specialized Skills
Gain expertise in specific areas of medical research, such as oncology, cardiology, or infectious diseases.
Learn about regulatory requirements and ethical standards in medical research.
Stay Current with Industry Trends
Read industry journals, such as The Lancet and JAMA, to keep abreast of the latest research findings.
Follow thought leaders and experts on social media platforms like LinkedIn and Twitter.
Leverage Technology
Use advanced research software and tools to enhance your data analysis and reporting capabilities.
Familiarize yourself with electronic data capture (EDC) systems and clinical trial management systems (CTMS).
The Impact of Medical Research Consultants on Healthcare
Medical research consultants play a pivotal role in advancing healthcare by contributing to the development of new treatments, improving patient care, and shaping health policies. Their expertise is essential in translating research findings into practical applications that benefit society.
Contributions to Medical Advancements
Medical research consultants have been instrumental in several key advancements, including:
Drug Development: Assisting in the creation of new medications and therapies.
Clinical Guidelines: Helping to establish evidence-based guidelines for patient care.
Public Health Initiatives: Supporting initiatives to prevent and control diseases.
Enhancing Patient Outcomes
By ensuring that research studies are scientifically rigorous and ethically sound, medical research consultants help improve patient outcomes. Their work leads to the development of safer and more effective treatments, ultimately enhancing the quality of care.
Shaping Health Policies
Medical research consultants provide valuable insights that inform health policies and regulations. Their expertise helps ensure that policies are based on the latest scientific evidence and best practices, promoting public health and safety.
Challenges Faced by Medical Research Consultants
While the role of a medical research consultant is rewarding, it also comes with its challenges. Understanding these challenges can help aspiring consultants prepare for the demands of the job.
Navigating Regulatory Requirements
Medical research is heavily regulated, and consultants must navigate complex regulatory landscapes. Staying informed about the latest regulations and ensuring compliance can be challenging but is essential for conducting ethical and legal research.
Managing Ethical Considerations
Ethical considerations are paramount in medical research. Consultants must ensure that studies are conducted ethically, with the rights and well-being of participants protected. This requires a deep understanding of ethical principles and guidelines.
Balancing Multiple Projects
Medical research consultants often juggle multiple projects simultaneously. Effective time management and organizational skills are crucial to handle the workload and meet deadlines.
Adapting to Technological Advances
The rapid pace of technological advancements in medical research requires consultants to continuously update their skills and knowledge. Staying current with the latest tools and methodologies is vital for success in this field.
Section III: Key Takeaways for Aspiring Medical Research Consultants
For those looking to embark on a career as a medical research consultant, here are some key takeaways to keep in mind:
Educational and Professional Development
Invest in Education: Pursue relevant degrees and certifications to build a strong foundation in medical research.
Continuous Learning: Stay updated with the latest research, technologies, and industry trends.
Networking and Collaboration
Join Professional Organizations: Engage with industry groups to expand your network and access resources.
Collaborate with Experts: Work with experienced professionals to gain insights and mentorship.
Skills and Competencies
Develop Technical Skills: Gain proficiency in research methodologies, data analysis, and regulatory compliance.
Enhance Soft Skills: Improve communication, problem-solving, and project management abilities.
Industry Trends and Opportunities
Stay Informed: Follow industry news and developments to identify new opportunities and areas of growth.
Leverage Technology: Use advanced tools and software to streamline research processes and enhance productivity.
Conclusion
Medical research consultants play a vital role in advancing healthcare by bridging the gap between research and practice. They contribute to the development of new treatments, improve patient care, and shape health policies. Aspiring medical research consultants should focus on gaining relevant education, building a strong professional network, and continuously updating their skills to succeed in this dynamic and rewarding field.
References
Clinical Laboratory Practice Certification
Good Clinical Laboratory Practice (GCLP)
Good Clinical Laboratory Practice (GCLP) certification is an essential component in the realm of clinical research and laboratory management. It ensures that laboratories operate under strict quality standards, which guarantees the reliability and integrity of data produced. This certification not only enhances the credibility of a laboratory but also plays a critical role in protecting public health by ensuring the accuracy of clinical trials and research.
What is GCLP?
Good Clinical Laboratory Practice (GCLP) combines principles of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) to ensure that laboratories conducting clinical trials produce reliable, reproducible, and high-quality data. GCLP guidelines cover all aspects of laboratory operations, from sample collection and analysis to data recording and reporting.
Importance of GCLP Certification
GCLP certification is vital for several reasons:
Quality Assurance: Ensures that laboratory processes and data are consistent and reproducible.
Regulatory Compliance: Meets the regulatory requirements set by authorities such as the FDA and EMA.
Data Integrity: Maintains the accuracy and integrity of data used in clinical research.
Risk Management: Reduces the risk of errors and non-compliance, which can lead to costly delays or failures in clinical trials.
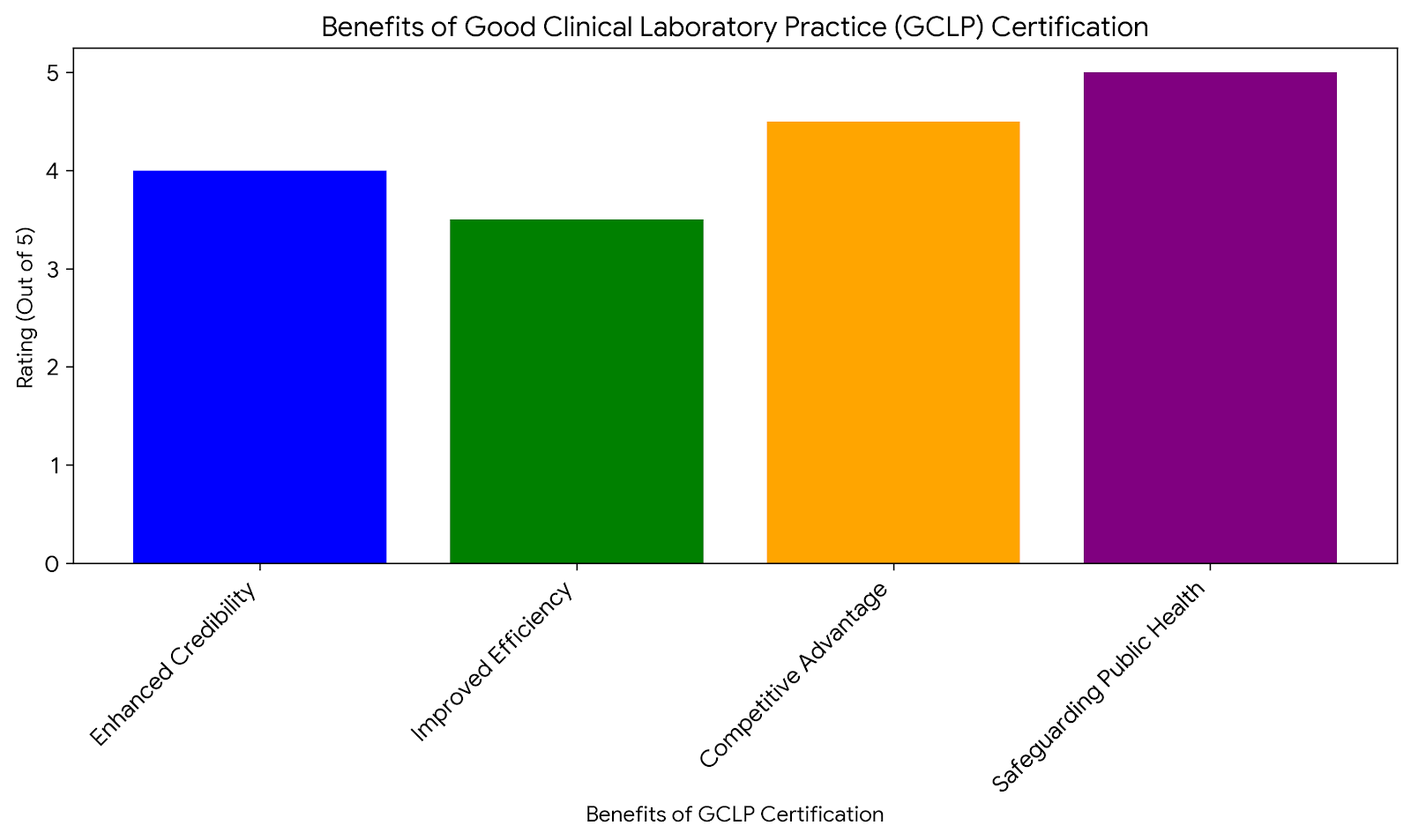
Enhanced Credibility and Trust
Obtaining GCLP certification significantly enhances a laboratory's credibility. It demonstrates a commitment to maintaining high standards and builds trust with sponsors, regulatory bodies, and the public.
Improved Laboratory Efficiency
Implementing GCLP guidelines streamlines laboratory processes, leading to increased efficiency and productivity. Standardized procedures reduce the likelihood of errors, saving time and resources in the long run.
Competitive Advantage
GCLP-certified laboratories are often preferred by sponsors and clients in the pharmaceutical and biotechnology industries. This certification can provide a competitive edge, attracting more business opportunities.
Safeguarding Public Health
By ensuring the accuracy and reliability of clinical data, GCLP certification plays a crucial role in safeguarding public health. Reliable data is essential for developing effective treatments and therapies.
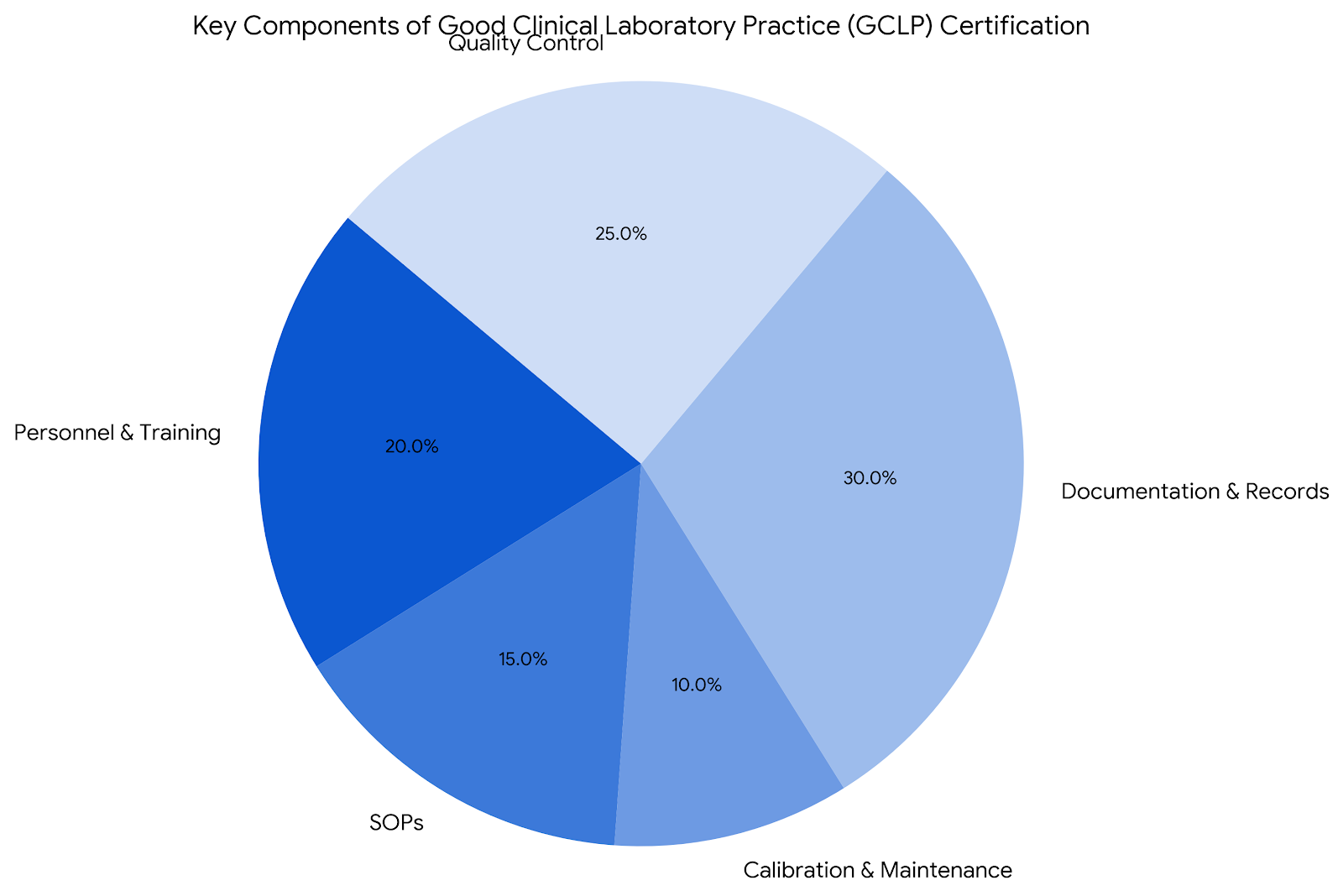
Key Components of GCLP Certification
Personnel and Training
Ensuring that laboratory personnel are adequately trained is a cornerstone of GCLP certification. Continuous training programs keep staff updated on the latest techniques and regulatory requirements.
Standard Operating Procedures (SOPs)
SOPs are detailed, written instructions to achieve uniformity in the performance of a specific function. They are crucial for maintaining consistency and quality in laboratory processes.
Equipment Calibration and Maintenance
Regular calibration and maintenance of laboratory equipment are essential to ensure accurate and reliable results. GCLP guidelines require documented proof of equipment performance.
Documentation and Records
Accurate documentation is fundamental to GCLP compliance. This includes detailed records of all laboratory activities, from sample collection to data analysis and reporting.
Quality Control and Assurance
Implementing robust quality control measures ensures that laboratory results are consistent and reliable. Quality assurance processes verify that all aspects of laboratory operations meet the required standards.
Implementing GCLP in Your Laboratory
Initial Assessment
Conducting an initial assessment of current laboratory practices against GCLP standards is the first step towards implementation. This helps identify gaps and areas for improvement.
Training and Education
Investing in comprehensive training programs for laboratory staff is crucial. This ensures that everyone is aware of GCLP guidelines and understands their role in maintaining compliance.
Developing SOPs
Creating detailed SOPs for all laboratory processes is essential. These should be regularly reviewed and updated to reflect any changes in practices or regulations.
Regular Audits
Conducting regular internal and external audits helps ensure ongoing compliance with GCLP standards. Audits can identify potential issues before they become significant problems.
Continuous Improvement
GCLP implementation is an ongoing process. Continuous improvement initiatives, such as feedback loops and process reviews, help maintain high standards and adapt to new challenges.
The Impact of GCLP Certification on Clinical Research
Ensuring Data Integrity
One of the most significant impacts of GCLP certification is the assurance of data integrity. Reliable data is the foundation of clinical research, and GCLP ensures that data is accurate, consistent, and reproducible.
Enhancing Patient Safety
By maintaining high standards of laboratory practice, GCLP certification enhances patient safety. Accurate and reliable data is crucial for developing safe and effective treatments.
Facilitating Regulatory Approval
GCLP-certified laboratories are more likely to meet the stringent requirements of regulatory authorities. This can expedite the approval process for new treatments and therapies, bringing them to market faster.
Boosting Research Efficiency
Standardized procedures and robust quality control measures streamline laboratory operations, leading to increased efficiency and productivity in clinical research.
Laboratory Information Management Systems (LIMS)
Laboratory Information Management Systems (LIMS) play a vital role in achieving GCLP compliance. These systems automate data collection, management, and reporting, ensuring accuracy and traceability.
Automation and Robotics
The integration of automation and robotics in laboratory processes reduces the likelihood of human error and increases efficiency. Automated systems can handle repetitive tasks, freeing up staff for more complex activities.
Data Analytics and AI
Advanced data analytics and artificial intelligence (AI) tools can enhance data analysis and interpretation, leading to more accurate and insightful results. These technologies support GCLP compliance by ensuring data integrity and reliability.
Evolving Standards and Guidelines
As scientific research and technology continue to advance, GCLP standards and guidelines will evolve. Staying updated with these changes is essential for maintaining compliance and ensuring the highest quality of laboratory practice.
Global Harmonization
Efforts towards global harmonization of GCLP standards are underway. This will facilitate international collaboration and streamline the regulatory approval process for new treatments and therapies.
Emphasis on Data Security
With the increasing amount of data generated in clinical research, data security will become a more significant focus. GCLP guidelines will likely include more stringent requirements for protecting sensitive information.
Final Thought
Good Clinical Laboratory Practice (GCLP) certification is a cornerstone of quality and reliability in clinical research. By ensuring high standards of laboratory practice, GCLP certification enhances credibility, efficiency, and data integrity. Implementing GCLP guidelines is an ongoing process that requires continuous improvement and adaptation to new challenges. As technology and standards evolve, staying updated with GCLP requirements will be crucial for maintaining compliance and ensuring the highest quality of clinical research.
References
ICH GCP Guidelines - ICH Official Website
FDA Guidelines on GCLP - FDA Official Website
EMA GCP Compliance - EMA Official Website
WHO GCLP Guidelines - WHO Official Website
Laboratory Information Management Systems (LIMS) - LabWare
Quality Assurance in Clinical Laboratories - Journal of Clinical Laboratory Analysis
Clinical Research Fast Track Salar
Clinical Research Careers
The field of clinical research offers a rewarding career path for those interested in advancing medical knowledge and patient care. Clinical researchers play a pivotal role in the development of new medications, therapies, and medical devices. These professionals ensure that clinical trials are conducted safely, ethically, and effectively, making significant contributions to the health industry.
Why Choose a Career in Clinical Research?
Clinical research careers are not only fulfilling but also offer substantial financial rewards. As the demand for new treatments and medical advancements grows, so does the need for qualified clinical research professionals. This demand is reflected in the competitive salaries offered in this field.
Moreover, a career in clinical research provides:
Job Security: With the continuous development of new treatments, the need for clinical research professionals remains steady.
Professional Growth: Opportunities for advancement and specialization in various areas of clinical research.
Impactful Work: Contributing to the development of life-saving treatments and improving patient outcomes.
Understanding Clinical Research Fast Track Programs
Clinical research fast track programs are designed to accelerate the training process for aspiring clinical researchers. These programs provide intensive, focused training, allowing individuals to quickly gain the skills and knowledge necessary to enter the field.
Key Features of Fast Track Programs
Accelerated Learning: Fast track programs condense the curriculum into a shorter time frame without sacrificing the quality of education.
Comprehensive Training: These programs cover all essential aspects of clinical research, including regulatory requirements, clinical trial design, and data management.
Practical Experience: Many fast track programs include hands-on training and internships to provide real-world experience.
Benefits of Enrolling in a Fast Track Program
Quick Entry into the Workforce: Accelerated programs enable students to start their careers sooner.
High Earning Potential: Graduates of fast track programs can quickly begin earning competitive salaries.
Increased Job Opportunities: Comprehensive training and practical experience make graduates highly desirable to employers.
Clinical Research Fast Track Salary Overview
One of the primary attractions of a career in clinical research is the potential for high earnings. Salaries in this field can vary widely based on factors such as experience, education, and geographic location.
Factors Influencing Salary
Several key factors influence the salary of clinical research professionals:
Experience: More experienced researchers typically command higher salaries.
Education: Advanced degrees and specialized certifications can significantly boost earning potential.
Location: Salaries can vary depending on the cost of living and demand for clinical research professionals in different regions.
Employer Type: Positions in pharmaceutical companies, contract research organizations (CROs), and academic institutions may offer different salary scales.
Salary Ranges for Various Positions
The following table provides an overview of typical salary ranges for different positions within clinical research:
Growing Demand for Clinical Research Professionals
The clinical research industry is experiencing robust growth, driven by the increasing number of clinical trials and the need for innovative medical solutions. This growth translates into a strong job market and competitive salaries for clinical research professionals.
Trends in Clinical Research Salaries
Recent trends indicate that salaries in clinical research are on the rise. Factors contributing to this trend include:
Increased Funding for Research: Governments and private organizations are investing heavily in medical research, leading to higher salaries.
Technological Advancements: The integration of new technologies in clinical trials requires skilled professionals, driving up salaries.
Globalization of Clinical Trials: The expansion of clinical trials to new markets increases demand for qualified researchers worldwide.
Regional Salary Differences
Salaries for clinical research professionals can vary significantly by region. For example:
United States: Clinical researchers in the U.S. typically earn higher salaries compared to their counterparts in other countries due to the high demand and cost of living.
Europe: Salaries in Europe vary widely, with countries like Switzerland and Germany offering higher pay rates.
Asia: Emerging markets in Asia, such as China and India, are seeing rapid growth in clinical research, leading to competitive salaries.
Obtaining Advanced Certifications
Earning advanced certifications can significantly enhance your career prospects and salary potential. Key certifications include:
Certified Clinical Research Professional (CCRP): Offered by the Society of Clinical Research Associates (SOCRA).
Clinical Research Coordinator (CRC): Provided by the Association of Clinical Research Professionals (ACRP).
Clinical Data Manager (CDM): Certification through the Society for Clinical Data Management (SCDM).
Continuing Education and Training
Staying current with the latest developments in clinical research is crucial for career advancement. Consider the following options:
Advanced Degrees: Pursuing a master's or doctoral degree in clinical research or a related field.
Professional Development Courses: Enrolling in specialized courses to enhance specific skills.
Industry Conferences and Workshops: Attending events to network and stay informed about industry trends.
Conclusion
A career in clinical research offers not only the opportunity to contribute to groundbreaking medical advancements but also the potential for a lucrative salary. With the right training, certifications, and commitment to continuous learning, clinical research professionals can enjoy a rewarding and financially secure career.
By understanding the factors that influence salaries and taking proactive steps to enhance your qualifications, you can position yourself for success in this dynamic and growing field.
References
Exploring Non-Clinical Opportunities for Physicians
Understanding the Appeal of Non-Clinical Roles
The medical field has long been revered for its dedication to patient care and medical advancement. However, the traditional path of a physician often involves long hours, high stress, and significant personal sacrifice. In recent years, a growing number of physicians have sought alternative career paths that utilize their medical expertise while offering a different lifestyle. Non-clinical opportunities provide a viable option for those looking to transition out of direct patient care without leaving the medical field altogether. In this blog, we will explore various non-clinical roles available to physicians, discuss the benefits and challenges of these careers, and provide guidance on making the transition successfully.
Work-Life Balance
One of the most compelling reasons physicians seek non-clinical roles is the potential for improved work-life balance. Clinical practice often demands long hours, night shifts, and being on call, which can take a toll on personal life and health. Non-clinical roles typically offer more regular hours and greater flexibility.
Diverse Career Opportunities
Non-clinical careers span a wide range of industries, including pharmaceuticals, healthcare administration, medical writing, and consulting. These roles allow physicians to leverage their medical knowledge in new and exciting ways.
Continued Use of Medical Expertise
Many physicians worry that leaving clinical practice means abandoning their medical training. However, non-clinical roles often require a deep understanding of medical concepts and patient care, allowing physicians to continue utilizing their expertise.
Types of Non-Clinical Opportunities
Healthcare Administration
Healthcare administration is a popular choice for physicians seeking non-clinical roles. These positions involve overseeing healthcare facilities, managing budgets, and improving patient care delivery systems. Key roles include:
Hospital Administrator
Medical Director
Chief Medical Officer
Pharmaceutical and Biotechnology Industries
The pharmaceutical and biotechnology industries offer various roles for physicians, including:
Medical Science Liaison
Clinical Research Scientist
Regulatory Affairs Specialist
Medical Writing and Communications
Physicians with strong writing skills may find rewarding careers in medical writing and communications. These roles include:
Medical Writer
Medical Editor
Healthcare Communications Specialist
Consulting
Consulting provides an opportunity for physicians to offer their expertise to healthcare organizations, insurance companies, and other entities. Roles in this field include:
Healthcare Consultant
Management Consultant
Medical Advisor
Education and Training
Physicians can also transition into roles focused on education and training, such as:
Medical School Faculty
Continuing Medical Education (CME) Provider
Medical Training Program Director
Benefits and Challenges of Non-Clinical Careers
Benefits
Improved Work-Life Balance: Non-clinical roles often come with regular hours and greater flexibility, allowing for a better work-life balance.
Diverse Career Opportunities: The variety of non-clinical roles means physicians can find a position that matches their interests and skills.
Continued Use of Medical Expertise: Many non-clinical positions still require a strong medical background, allowing physicians to leverage their knowledge in new ways.
Challenges
Transitioning Skills: Moving from a clinical to a non-clinical role can be challenging, as it often requires developing new skills and knowledge.
Financial Considerations: Some non-clinical roles may offer lower salaries compared to clinical practice, although this varies widely.
Identity and Satisfaction: Physicians may struggle with the change in identity and job satisfaction when leaving direct patient care.
Making the Transition to a Non-Clinical Career
Assessing Your Interests and Skills
Before making a transition, it’s important to assess your interests and skills. Consider what aspects of your clinical work you enjoy and how they might translate to a non-clinical role. Reflect on your strengths and areas where you may need additional training or education.
Networking and Research
Networking is crucial when transitioning to a non-clinical career. Connect with colleagues who have made similar transitions, attend industry conferences, and join professional organizations. Research potential roles and employers to gain a better understanding of the opportunities available.
Gaining Additional Qualifications
Some non-clinical roles may require additional qualifications or certifications. For example, a physician looking to move into healthcare administration might benefit from earning a Master of Business Administration (MBA) or a Master of Health Administration (MHA).
Building a Strong Resume and Online Presence
A strong resume and online presence are essential when applying for non-clinical roles. Highlight your medical expertise, relevant skills, and any additional qualifications. Consider creating a LinkedIn profile to connect with industry professionals and showcase your experience.
Preparing for Interviews
Prepare for interviews by practicing responses to common questions and researching the company or organization. Be ready to discuss how your medical background and skills make you a good fit for the role. Emphasize your ability to adapt and learn new things quickly.
Key Considerations for Physicians Exploring Non-Clinical Careers
Financial Implications
Transitioning to a non-clinical career can have financial implications. While some non-clinical roles offer competitive salaries, others may pay less than clinical practice. It's important to evaluate your financial situation and consider how a change in salary might impact your lifestyle.
Emotional and Psychological Impact
Leaving clinical practice can be emotionally challenging. Physicians often identify strongly with their role in patient care, and transitioning to a non-clinical career can feel like a loss of identity. It's important to address these feelings and seek support from peers, mentors, or a professional counselor if needed.
Continuous Learning and Adaptation
Non-clinical roles may require continuous learning and adaptation. Physicians should be prepared to acquire new skills, stay updated on industry trends, and be open to new experiences. This commitment to ongoing professional development can enhance job satisfaction and career success.
Successful Examples of Physicians in Non-Clinical Roles
Dr. John Smith: Transitioned from clinical practice to a leadership role in healthcare administration, becoming a Chief Medical Officer at a major hospital.
Dr. Jane Doe: Moved into the pharmaceutical industry as a Medical Science Liaison, leveraging her medical expertise to bridge the gap between research and clinical practice.
Dr. Richard Roe: Pursued a career in medical writing, authoring research papers and medical textbooks that contribute to the advancement of medical knowledge.
Tips for a Smooth Transition
Seek Mentorship: Find a mentor who has successfully transitioned to a non-clinical role. Their guidance and insights can be invaluable.
Stay Connected: Maintain connections with your medical peers and professional networks. This can provide support and potential job leads.
Focus on Transferable Skills: Highlight transferable skills such as leadership, problem-solving, and communication in your job applications.
Be Patient: The transition to a non-clinical career may take time. Be patient and persistent in your job search and professional development efforts.
Conclusion
Exploring non-clinical opportunities can be a rewarding career path for physicians seeking to leverage their medical expertise in new ways while achieving a better work-life balance. With careful planning, networking, and a willingness to learn, physicians can successfully transition to non-clinical roles that offer both personal and professional fulfillment. By considering the benefits and challenges, assessing their interests and skills, and seeking mentorship, physicians can navigate this transition smoothly and find new ways to contribute to the healthcare industry.
References
American College of Healthcare Executives. (2023). Healthcare Administration Careers. Retrieved from ACHE
Pharmaceutical Research and Manufacturers of America. (2023). Careers in the Pharmaceutical Industry. Retrieved from PhRMA
American Medical Writers Association. (2023). Medical Writing as a Career. Retrieved from AMWA
Society of Healthcare Consultants. (2023). Consulting Careers for Physicians. Retrieved from SHC
American Association for Physician Leadership. (2023). Physician Leadership and Education. Retrieved from AAPL
Institute for Continuing Medical Education. (2023). Opportunities in CME. Retrieved from ICME
By following these guidelines and considering the various aspects of non-clinical careers, physicians can make informed decisions and find fulfilling roles that align with their professional goals and personal aspirations.
Clinical research remote positions
The Evolution of Remote Clinical Research
In recent years, the landscape of clinical research has seen a significant shift towards remote positions. This transformation is driven by advancements in technology, changing workplace dynamics, and the need for greater flexibility. In this blog, we will explore the key aspects of remote positions in clinical research, their benefits, challenges, and future prospects.
A New Era in Clinical Research
The COVID-19 pandemic accelerated the adoption of remote work across various industries, including clinical research. This shift has enabled clinical trials to continue with minimal disruption, ensuring that critical research can progress without compromising on safety or data integrity. Remote clinical research positions have become more common, allowing professionals to work from anywhere in the world.
Key Drivers of Remote Clinical Research
Several factors have contributed to the rise of remote positions in clinical research:
Technological Advancements: Improved communication tools, electronic data capture (EDC) systems, and remote monitoring technologies have made it easier to conduct clinical research from a distance.
Regulatory Flexibility: Regulatory bodies, such as the FDA, have provided guidance on decentralized clinical trials, paving the way for remote research methods.
Cost Efficiency: Remote work reduces overhead costs associated with maintaining physical office spaces and travel expenses.
Access to a Broader Talent Pool: Organizations can tap into a global talent pool, attracting skilled professionals from diverse geographic locations.
Benefits of Remote Positions in Clinical Research
Enhanced Flexibility and Work-Life Balance
Remote positions offer clinical research professionals the flexibility to work from home or any location of their choice. This flexibility enhances work-life balance, reducing stress and increasing job satisfaction.
Flexibility: Professionals can create their own schedules, leading to a better work-life balance.
Reduced Commute: Eliminating the daily commute saves time and money, contributing to a healthier lifestyle.
Increased Productivity: Many professionals find that they are more productive when working in a comfortable and familiar environment.
Access to Global Talent
Remote positions break down geographic barriers, allowing organizations to hire the best talent from around the world. This diversity brings fresh perspectives and innovative solutions to clinical research challenges.
Diverse Expertise: Access to a wider pool of candidates with varied expertise and backgrounds.
Global Collaboration: Enhanced collaboration across different time zones, leading to round-the-clock progress on research projects.
Competitive Advantage: Organizations can stay ahead of the competition by leveraging the skills and knowledge of a global workforce.
Challenges and Solutions in Remote Clinical Research
Overcoming Communication Barriers
Effective communication is crucial in remote clinical research. Without face-to-face interactions, it can be challenging to ensure clear and consistent communication among team members.
Regular Check-ins: Implement regular virtual meetings to keep everyone aligned and address any issues promptly.
Collaboration Tools: Utilize advanced collaboration tools, such as Slack, Microsoft Teams, and Zoom, to facilitate seamless communication.
Clear Documentation: Maintain thorough and accessible documentation to ensure everyone has the information they need.
Ensuring Data Security and Compliance
Remote work can pose challenges to data security and regulatory compliance. It is essential to implement robust measures to protect sensitive information and adhere to regulatory standards.
Secure Access: Use secure, encrypted connections for accessing and sharing data.
Training and Awareness: Provide regular training on data security best practices and regulatory requirements.
Compliance Monitoring: Implement continuous monitoring to ensure compliance with industry regulations.
Future Prospects of Remote Clinical Research Positions
Continued Growth and Innovation
The trend towards remote clinical research positions is expected to continue, driven by ongoing technological advancements and the need for flexible work arrangements. Organizations will increasingly adopt decentralized clinical trial models, leveraging remote monitoring and data collection technologies.
Embracing Hybrid Models
While remote work offers numerous benefits, some aspects of clinical research may still require in-person interactions. Hybrid models, combining remote and on-site work, will likely become more prevalent, offering the best of both worlds.
Investing in Remote Work Infrastructure
Organizations will need to invest in robust remote work infrastructure to support their teams effectively. This includes advanced communication tools, secure data management systems, and comprehensive training programs.
Conclusion
Remote positions in clinical research are transforming the industry, offering numerous benefits while presenting unique challenges. By embracing technological advancements and implementing effective communication and data security measures, organizations can successfully navigate this new landscape. The future of clinical research lies in the balance between remote and on-site work, ensuring flexibility, efficiency, and innovation.
For more insights and updates on clinical research trends, check out the following references:
FDA Guidance on Decentralized Clinical Trials
By staying informed and adapting to the evolving landscape, clinical research professionals can thrive in remote positions and contribute to groundbreaking advancements in medical science.
Clinical Trial Associate vs Clinical Research Associate
Understanding the Key Differences and Roles
Clinical research plays a crucial role in the advancement of medical knowledge and the development of new treatments and therapies. Within this field, the roles of Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs) are often discussed and sometimes confused. While both positions are vital to the success of clinical trials, they have distinct responsibilities and requirements.
In this comprehensive blog, we will explore the roles of CTAs and CRAs in depth, highlighting their differences, similarities, and the unique contributions each role makes to the clinical research process.
Clinical trials are the backbone of medical research, providing the necessary evidence to support new treatments, medications, and interventions. The success of these trials relies on a well-coordinated effort from various professionals, including Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs). Understanding the distinct roles and responsibilities of these positions is essential for anyone considering a career in clinical research or looking to collaborate effectively within a clinical trial team.
What is a Clinical Trial Associate (CTA)?
A Clinical Trial Associate (CTA) is a key member of the clinical trial team, responsible for providing administrative and operational support throughout the duration of a clinical study. CTAs are often involved in the early stages of the trial, assisting with the preparation and organization of essential documents and ensuring that all regulatory requirements are met.
Key Responsibilities of a CTA
Document Management: CTAs are responsible for the creation, maintenance, and organization of trial master files (TMFs), ensuring that all essential documents are properly filed and accessible.
Regulatory Compliance: They assist in the preparation and submission of regulatory documents, ensuring compliance with local, national, and international regulations. For more information on the roles and responsibilities, refer to the Clinical Trial Associate Job Description on Betterteam.
Communication: CTAs facilitate communication between the clinical trial team, study sites, and external stakeholders, ensuring that all parties are informed and updated on trial progress.
Administrative Support: They provide general administrative support, including scheduling meetings, preparing meeting minutes, and coordinating travel arrangements for the clinical trial team.
Data Entry and Management: CTAs may be involved in the entry and management of trial data, ensuring accuracy and completeness of data collected during the study.
Skills and Qualifications Required for a CTA
Educational Background: A bachelor's degree in life sciences, healthcare, or a related field is typically required. Some positions may accept candidates with an associate degree or relevant work experience.
Attention to Detail: CTAs must have a keen eye for detail, ensuring that all documents and data are accurate and complete.
Organizational Skills: Strong organizational skills are essential for managing multiple tasks and ensuring that all trial-related documents are properly maintained.
Communication Skills: Excellent written and verbal communication skills are necessary for effectively coordinating with study sites and other stakeholders.
Technical Proficiency: Proficiency in Microsoft Office and clinical trial management systems (CTMS) is often required. Additional insights can be found in the Roles and Responsibilities of a Clinical Trial Associate on JobHero.
What is a Clinical Research Associate (CRA)?
A Clinical Research Associate (CRA) plays a more hands-on role in the clinical trial process, primarily responsible for monitoring the progress of clinical studies and ensuring that they are conducted in accordance with regulatory requirements and study protocols. CRAs often work directly with study sites, conducting site visits and ensuring that data collected is accurate and complete.
Key Responsibilities of a CRA
Site Monitoring: CRAs conduct regular site visits to monitor the progress of clinical trials, ensuring that study sites adhere to the protocol and regulatory requirements. Detailed information about CRA roles can be found on ProClinical.
Data Verification: They review and verify data collected during the trial, ensuring its accuracy and completeness.
Training and Support: CRAs provide training and support to site staff, ensuring that they are knowledgeable about the study protocol and procedures.
Regulatory Compliance: They ensure that all regulatory requirements are met and that study sites are prepared for audits and inspections.
Issue Resolution: CRAs identify and resolve any issues or discrepancies that arise during the trial, ensuring that the study stays on track and within compliance.
Skills and Qualifications Required for a CRA
Educational Background: A bachelor's degree in life sciences, healthcare, or a related field is typically required. Advanced degrees or certifications (e.g., CCRA) can be advantageous. For further career guidance, see the Clinical Research Associate Career Profile on Study.com.
Clinical Experience: Prior experience in clinical research or a related field is often required, with many CRAs having a background in nursing, pharmacy, or other healthcare professions.
Analytical Skills: Strong analytical skills are essential for reviewing and verifying trial data.
Communication Skills: Excellent communication skills are necessary for effectively interacting with study sites and other stakeholders.
Travel Flexibility: CRAs often travel frequently for site visits, so flexibility and willingness to travel are important.
Differences Between CTAs and CRAs
While both CTAs and CRAs play critical roles in clinical trials, their responsibilities and focus areas differ significantly.
Scope of Work
CTAs: Primarily focused on administrative and operational support, ensuring that all documents and regulatory requirements are in place. Their work is often more office-based.
CRAs: Focused on site monitoring and data verification, ensuring that trials are conducted according to protocol and regulatory standards. Their work often involves significant travel and direct interaction with study sites. For more comparison details, visit Clinical Research Associate vs. Clinical Research Coordinator on Clinical Professionals.
Interaction with Study Sites
CTAs: Limited direct interaction with study sites, primarily facilitating communication and providing administrative support from a central location.
CRAs: Regularly interact with study sites, conducting site visits, providing training, and resolving issues directly with site staff.
Data Handling and Management
CTAs: May be involved in data entry and management, ensuring that data is accurately recorded and maintained.
CRAs: Responsible for verifying the accuracy and completeness of data collected during the trial, ensuring that it meets regulatory and protocol standards.
Similarities Between CTAs and CRAs
Despite their differences, CTAs and CRAs share some commonalities in their roles and contributions to clinical trials.
Contribution to Clinical Trials
Both CTAs and CRAs play essential roles in ensuring the success of clinical trials, contributing to the overall goal of advancing medical knowledge and improving patient care. For more on clinical trials, see Clinical Trials: What You Need to Know on the FDA website.
Regulatory Compliance
Both positions require a strong understanding of regulatory requirements and guidelines, ensuring that clinical trials are conducted ethically and in compliance with all applicable regulations.
Career Pathways for CTAs and CRAs
Both CTAs and CRAs have opportunities for career advancement and professional development within the field of clinical research.
Career Advancement Opportunities
CTAs: With experience and additional training, CTAs can advance to positions such as Clinical Research Coordinator (CRC), Regulatory Affairs Specialist, or Project Manager.
CRAs: CRAs can advance to senior CRA positions, Lead CRA, Clinical Project Manager, or Clinical Operations Manager. For career guidance, refer to How to Become a Clinical Research Associate on WikiHow.
Professional Development
Both CTAs and CRAs can benefit from continued education and certification programs, such as the Clinical Research Coordinator (CRC) certification for CTAs or the Certified Clinical Research Associate (CCRA) certification for CRAs. For more on training and certifications, see Clinical Research Training and Certification on the AACR website.
Final Thoughts
The roles of Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs) are both vital to the success of clinical trials, each contributing in unique ways to the advancement of medical research. While CTAs provide essential administrative and operational support, ensuring that all regulatory requirements are met, CRAs take on a more hands-on role, monitoring study sites and verifying data accuracy. Understanding the distinct responsibilities and requirements of these roles is essential for anyone considering a career in clinical research or looking to collaborate effectively within a clinical trial team.
In the dynamic and ever-evolving field of clinical research, both CTAs and CRAs have the opportunity to make significant contributions to the development of new treatments and therapies, ultimately improving patient care and advancing medical knowledge.
CCRPS Reviews: Maria Lopez's Journey with CCRPS Certification: From Physician to Chief Medical Officer at a Specialized CRO
Before CCRPS Maria Lopez was an experienced physician with a background in medical devices, looking to expand her role within clinical research. After CCRPS Maria ascended to the role of Chief Medical Officer at a CRO in Columbia, specializing in medical devices, significantly enhancing her expertise in clinical research operations.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Maria's insights provide a profound view of the impact that the CCRPS certification program has had on her professional capabilities and her organization's services.
Key Takeaways
● Maria appreciated how the course was structured, which allowed her to develop a deep understanding of clinical research administration, particularly in the context of medical devices.
● The inclusion of templates and structured guidance within the course enabled Maria to streamline her workflow and implement standardized procedures at her CRO.
● The focus on quality and safety in the course resonated with Maria’s values as a physician, empowering her to uphold high standards in her organization's research practices.
Engagement and Professional Development:
● The course's flexible, self-paced format was perfectly suited to Maria’s busy schedule, allowing her to engage deeply with the material and apply the knowledge effectively in her role.
Closing Thoughts:
● Maria's experience with the CCRPS certification underscores its value not just in educational enrichment but also in practical application within the clinical research industry. Her story is a testament to the program's role in advancing careers and enhancing the quality of research practices globally.
Recommendations to Colleagues:
● Maria strongly recommends the CCRPS course to her peers, emphasizing its comprehensive coverage of clinical trials, its practical utility, and the continuous access to updated content, ensuring that learners stay informed of the latest industry standards and regulations.
Career Advancement:
● The CCRPS certification was pivotal in Maria’s promotion to Chief Medical Officer, where she now plays a crucial role in expanding her company’s services to include clinical research for medical devices, demonstrating the direct career benefits of the program.
Insights for Future Improvement:
● Maria suggested enhancements to the course, including more interactive teaching methods beyond slide reading and better follow-up on participant inquiries to strengthen the learning experience.
@0:00 Courtney Fulkerson: "All right. First of all, thank you for meeting with me today and we're pretty much just going to kind of go through some questions and get your feedback on the program and things that you like about it, things you didn't and pretty much just to tailor the program from here on out like any feedback."
@1:15 Maria Lopez: "It was really important for me, the part where we're at it. I mean, you usually know the basics of the history of political research, but it was like a really wide and long, it gave me a lot of information about it in a timeline. It was many things I didn't know. And now I have a wider structure about it. It was really from the beginning was like, wow, they usually go really fast through ethics, but it was really important."
@2:30 Maria Lopez: "The other thing that I loved is you gave us a lot of templates and for example, letter structures, then you can start using them that when you now have a file of papers that I don't have to have a changed name, the log. Oh, I've got a little bit more of my devices, but not only the knowledge, but now have a lot of papers that are always ready to start going on. So that is that was amazing. That is the, this is the only course I ever done that I really find that those many times and stuff."
@3:45 Courtney Fulkerson: "Yeah, it's good to hear just any really point of view. And I feel like as long as it was useful and you're able to kind of talk about like the way you've been able to use it, which I know you have a little bit, but we're grateful for any feedback."
@4:50 Maria Lopez: "I was really interested in quality that is like the main thing we are really into it. So quality was when I read what I went over the course, me and my boss, we were ready. It was like, OK, we need this to be like really into quality. Because our CRO, we all of us, I mean, our doctors, we're all physicians, basically, we're physicians. So all everything around quality and safety, it's really important."
@6:10 Courtney Fulkerson: "Right, that's very, I mean, it's important. It's important in anything. But I mean, that's not too good that you found that within our course."
@7:22 Maria Lopez: "You know, me as a non, I mean, I speak my native language Spanish and Sometimes you get into those programs and you have many accents that are so hard, but you didn't. That was really good. I understood like everyone."
@8:55 Courtney Fulkerson: "Good."
@9:10 Maria Lopez: "I'm definitely coming back. I didn't download everything. And I just got it there. And the topics that I feel like not very strong, I've listened to them. Not like starting for the exam, but like listening to it. we were a podcast, listening to them and really getting into them."
CCRPS Reviews: Hannah Fischer's Career Growth with CCRPS Certification: From Grant Program Manager to Leading Clinical Trials at UCSF
Before CCRPS: Hannah Fischer Was A Grant Manager Struggling with Clinical Trial Coordination. After CCRPS She Elevated to Clinical Trial Leader at UCSF, Excelling in Trial Management and Grant Applications, Thanks to Our Clinical Trials Training.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Hannah Fischer’s feedback underscores the significant positive impact of the CCRPS certification program on her professional life. Her experience highlights the program’s effectiveness in enhancing participants' understanding of clinical trials, improving job performance, and facilitating career advancement. This testimonial serves as a strong endorsement of the program’s value to professionals in the clinical trials industry.
Key Takeaways
● Hannah had a very positive experience with the self-paced online format, which she found accommodating to her busy schedule.
● The content was comprehensive and directly applicable to her current role in managing clinical trials, significantly aiding her daily responsibilities and strategic approach.
● Successfully leveraged course content and certification to secure a promotion at work, underscoring the practical benefits and career enhancement potential of the program.
● Enthusiastically recommended the course to colleagues due to its thorough coverage of clinical trials from start to finish.
Recommendations to Colleagues:
● Hannah enthusiastically recommended the course to her colleagues due to its thorough coverage of clinical trials, highlighting its value in professional development within her field.
Learning Tools and Resources:
● Quizzes and the comprehensive final exam were highlighted as beneficial for reinforcing knowledge and ensuring thorough understanding of the material.
Career Advancement:
● The certification and the knowledge gained from the course played a crucial role in Hannah securing a promotion at work, illustrating the career enhancement potential of the program.
Course Structure and Content:
● She appreciated the self-paced online format, which was perfectly suited to her busy schedule, allowing her to integrate learning seamlessly with her professional responsibilities.
● The course provided a solid foundation in understanding clinical trials from start to finish, making complex processes understandable and manageable.
@0:00 - Courtney Fulkerson: "Congrats on successfully completing your CCRPS program, and thank you also for taking time to meet with me today. We're pretty much just going to go through some questions and answers just to have a conversation about basically how we can improve the program and then your experience with it and kind of where you're at right now in your professional career. Alright, and if you're ready, we'll go ahead and get started with some questions. Okay, could you share some of your favorite moments from the course that enriched your learning experience?"
@0:48 - Hannah Fischer: "I think that's very beneficial. It would be fun to think about, you know, what is needed in the workplace. It helped me. I worked in grants, so I'm the grant program manager for our department, and we are starting a clinical trial for one of our products. I didn't necessarily have a strong engagement and understanding of how everything works together. I have little pieces; I've done this before, but being on the grant side, having this understanding really helped me put together our new grant, our OC2 grant together, and also trying to help implement this trial with some of the events."
@1:35 - Courtney Fulkerson: "Right, well that's really good to hear. What sort of program were you looking for when you chose CCRPS and were you pleased with the content of the course once you got started?"
@2:12 - Hannah Fischer: "I was looking for something that fit my schedule, had a very busy schedule with us, so when I was looking I was looking for something that was self-paced, but also something that had a good foundation, so it explains everything from start to finish about what all is involved in clinical trials, so that it's not too overwhelming."
@4:12 - Courtney Fulkerson: "Would you consider revisiting the course for a refresher, and is there any specific information that you'd want to see within a refresher course?"
@4:25 - Hannah Fischer: "Yes, I can see how that could help with my job in clinical research. Integrating real-world scenarios would be helpful, just because it's nice to see how this knowledge is applied in the workplace."
@4:50 - Courtney Fulkerson: "And if someone from our program were to ask for recommendations for future editions, such as the 2025 edition coming up, would you be open to sharing a list of recommendations and potentially being featured as a course contributor on a future edition of the course?"
@5:05 - Hannah Fischer: "Absolutely. I'm all for continuing education and helping others. If I can help anyone, please let me help."
@5:19 - Courtney Fulkerson: "Can you share what factors motivated you to choose this course and how it stood out from other options that you might have considered?"
@5:41 - Hannah Fischer: "I was looking for something that was self-paced because of my busy schedule. I needed something that provided a thorough foundation, explaining clinical trials from start to finish, so it matched my needs well. CCRPS offered that, and it was easier to fit it into my schedule."
@6:02 - Courtney Fulkerson: "Great, it checked all your boxes then. Now, can you share about your professional journey prior to embarking on this course and how you envision the course contributing to your career growth?"
@6:26 - Hannah Fischer: "Yes. Before taking this course, I was involved in clinical trials administration and coordination but needed a deeper understanding to write clinical trial grants successfully. The comprehensive nature of this course really helped me improve my understanding, which is crucial for my work in managing grants and coordinating trials."
@7:01 - Courtney Fulkerson: "How long did it take you to complete the course and do you feel like the duration was adequate for your learning?"
@7:14 - Hannah Fischer: "I worked on it periodically over 2-3 months. It could be completed in 1 month if someone focused fully on it. The pace was good — the content was clearly explained and I didn't feel rushed."
@7:45 - Courtney Fulkerson: "After completing the course, how did you update your resume and enhance your professional profile?"
@8:06 - Hannah Fischer: "I added the CCRPS certification to my resume and LinkedIn profile. It gave me credibility and deeper knowledge that set me apart in my field. It's definitely helped in job interviews and discussions within my professional network."
@8:36 - Courtney Fulkerson: "How would you describe your experience with our learning platform? Did it facilitate a smooth and engaging learning process?"
@8:48 - Hannah Fischer: "The platform was easy to navigate and use. The content was accessible, and I could go back to review material as needed, which was very helpful for reinforcing knowledge."
@9:35 - Courtney Fulkerson: "And finally, what part of the course did you find most engaging and beneficial to your professional development?"
@9:48 - Hannah Fischer: "The quizzes were particularly helpful for reinforcing the material. The final exam was also very beneficial as it forced a comprehensive review of everything covered, which solidified my understanding and confidence in the subject matter."
@10:23 - Courtney Fulkerson: "What are some reasons you would recommend this course to others based on your experience?"
@10:30 - Hannah Fischer: "It covers clinical trials thoroughly from start to finish and is helpful for anyone involved in clinical trials. I've recommended it to colleagues because it provides a solid foundation and understanding of the clinical trial process, which is essential for anyone in this field."
@10:44 - Courtney Fulkerson: "And lastly, could you share how your career has advanced since completing the course?"
@10:58 - Hannah Fischer: "Since completing the course, I've been promoted at work. The knowledge I gained through the CCRPS program directly helped me secure a higher position where I now manage more critical aspects of clinical trials. It's been a significant boost to my career."
CCRPS Reviews: Scott Boyle: From CRO Intern to Regulatory Affairs Specialist Enhanced by ICH GCP Certification
Scott Boyle's Professional Advancement with CCRPS GCP Certification: From Intern at a CRO to Regulatory Affairs Specialist at UPenn
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Scott Boyle’s feedback underscoring the effectiveness of the GCP certification program in providing essential knowledge and skills for professionals transitioning into or advancing within regulatory affairs. His suggestions for bundling courses and adding detailed workflows indicate areas for potential enhancement that could benefit future learners. Overall, Scott’s experience with the course was highly positive, significantly impacting his professional development and career progression.
Key Takeaways
● Scott found the course straightforward to navigate and access, appreciating the professionalism of the website and the reasonable pricing.
● The self-paced nature of the course was beneficial, allowing Scott to fit the learning into his schedule effectively.
● The course content, especially the Code of Federal Regulations section, was directly applicable to Scott’s job in regulatory affairs, enhancing his confidence and competence.
● Completing the course helped Scott secure a job in regulatory affairs, attributing his success in part to the confidence gained from being GCP certified.
Professional Development:
● The GCP certification was a significant addition to Scott’s resume and LinkedIn profile, leading to increased visibility and job opportunities in clinical research.
Recommendations for Others:
● Scott would recommend the course to peers for its accessibility, affordability, and comprehensive coverage of essential GCP knowledge.
Content Satisfaction:
● The course covered essential GCP elements that were crucial for Scott’s role, particularly the detailed information on regulatory requirements which he found most engaging.
Learning Platform:
● Scott praised the learning platform for its user-friendliness and the ability to revisit the content anytime, which he found helpful for continuous learning.
@1:00 - Courtney Fulkerson:
"You're looking to get a certification or like what made you choose this specific one?"
@1:05 - Scott Boyle:
"It was exactly GCP. It was a great refresher learning course. It wasn't required as part of my current job now. They made me redo my GCP. But overall, I think it was a great refresher and it did help me prepare for my current job now."
@1:37 - Scott Boyle:
"I think maybe if there was like a bundle, like I could do like a GCP and a CRA kind of thing."
@1:48 - Scott Boyle:
"Combining two courses, yeah, because I would say some of them can definitely go hand in hand and others could definitely, I mean, all together work out pretty good."
@2:07 - Scott Boyle:
"So I currently work in regulatory affairs at UPenn. Maybe if there is a regulatory aspect of it, I'm a regulatory affairs specialist. Yeah. could be like a like a workflow process kind of thing like this is how every day is going to work for generalized regulatory affairs specialists. And then once you go to a company, it can vary depending on company policies."
@3:20 - Scott Boyle:
"And is there anything that you would like to see within that if you were to? I already have. I already went on the website recently, and I was just reading through it again about processes. again, the CRF, Title 21, overall, I'd say it was good taking class and what I like most about it is in my account. I can still go back or refer to it whenever I want. I like how it's a one-time thing, and there's not blocked by a bunch of different day walls."
@5:05 - Scott Boyle:
"Yes, I would."
@5:41 - Scott Boyle:
"Um, I would say the website looked the most professional out of all. And the GCP course was reasonably priced at very easy to access. I mean, I think it was like two Google searches and I got right there."
@6:02 - Scott Boyle:
"I do do others, but I think overall it was easy to use. It was very open with what you offered. And I think it was pretty fairly priced at $50."
@6:26 - Scott Boyle:
"So before I took this, I did an internship at a CRO company. And they were talking all about GCP and ICH. And it encouraged me to go out and get the certification. I just didn't know where to go. And going back to the same thing, as I said before, it was very easy to go to Google found this organization right away. And they gave me a pretty informational course and a certification to go along with it. that was very helpful."
@7:27 - Scott Boyle:
"Yeah, I loved that with self paste."
@7:45 - Scott Boyle:
"And then how has it enhanced your professional profile? I would say is it has helped me get my current job now. GCP certified on my resume, and then I even put it on my LinkedIn. And I have I noticed more people requesting to follow me in clinical research because they see some of my credentials."
@8:17 - Scott Boyle:
"I would say it overall was very good because like you said again, it was self-paced and there was an option to, if people wanted to, can like read faster, you could have the person reading speed it up to like times two or times five. That was a good process."
@9:35 - Courtney Fulkerson:
"Good. I'm glad that that made you feel confident and I feel like that is one of the biggest parts of clinical research so it's always good to know."
@10:30 - Scott Boyle:
"The top reasons I'd say is easy to navigate, you make your account, you pay a one-time fee, and then you can always go back to it, which I think is very, very good."
CCRPS Reviews: Justin Scott Brathwaite From a Startup Associate to a Senior Startup Specialist, Clinical Research Coordinator Graduate.
Justin Scott Brathwaite's Career Growth Through the CCRPS Coordinator Course Justin Scott Brathwaite advanced from a Startup Associate to a Senior Startup Specialist after completing the Clinical Research Coordinator course offered by CCRPS.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Justin Scott Brathwaite’s feedback emphasizing the course's strengths in providing comprehensive, relevant content at an affordable price. His career advancement post-course completion serves as a testament to the program's effectiveness in equipping participants with the necessary skills and knowledge for professional growth in clinical research.
Key Takeaways
● The broad knowledge gained from the course has bolstered Justin's confidence, particularly in his ability to understand and manage different roles within clinical research. This is pivotal as he aims to move into a project management role, indicating his readiness to handle more complex tasks and leadership duties.
● Since completing the course, Justin has advanced from a Startup Associate to a Senior Startup Specialist, and he is now taking on responsibilities related to project management. This progression demonstrates the tangible impact of the course on his career trajectory.
● Justin's willingness to recommend the course to others, especially due to its comprehensive content and affordability, underscores his positive evaluation of the program's value. This recommendation reflects his belief in the course's ability to provide foundational knowledge essential for newcomers to the field or those seeking a cost-effective way to enhance their skills.
● Justin's participation in the CRC course significantly improved his grasp of critical aspects such as regulatory standards, ICH guidelines, GCP, and SIV. This comprehensive understanding is crucial for his role in study start-up and aids in his professional efficacy.
Confidence in Professional Settings:
● The broad knowledge acquired from the course has enhanced Justin’s confidence, particularly in understanding different roles within clinical research, which supports his ambition to transition into a project management role..
Enrollment Motivation:
● He was attracted to the program through a LinkedIn advertisement, citing the affordability of the course compared to other options as a major factor in his decision to enroll.
Professional Impact and Career Growth:
● Since completing the course in 2020, Justin has advanced from a Startup Associate to a Senior Startup Specialist and is now taking on project management responsibilities. The course significantly contributed to his understanding of site operations and broadened his perspective, facilitating his career progression.
Course Value and Effectiveness:
● Justin praised the course for being comprehensive and cost-effective. He found the focus on site perspectives particularly enlightening as it aligned with his work in Startup Associate to Senior Startup Specialist..
Content Relevance:
● The sections on regulatory standards, International Council for Harmonisation (ICH) guidelines, Good Clinical Practice (GCP), and Site Initiation Visits (SIV) were highlighted as the most relevant and beneficial, directly aiding his professional duties.
@0:00 - Justin Scott Brathwaite: "Hey Courtney, how are you?"
@0:27 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "That's perfect. I'm just going to start recording on Zoom as well."
@0:39 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "How are you today?"
@0:40 - Justin Scott Brathwaite: "Doing well."
@1:42 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "Could you share some of your favorite moments from the course that enriched your learning experience?"
@1:49 - Justin Scott Brathwaite: "Yeah, I took the clinical research coordinator course. I work in study startup and it was really helpful to understand the coordinator's role since I do a lot of work with sites. I found the SIV, ICH, and GCP sections particularly helpful."
@2:45 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "So it was pretty comprehensive. Good. So you feel like it kind of covered a lot of bases and it was able to translate into what you’re doing now."
@3:10 - Justin Scott Brathwaite: "I was looking for something that was relatively cost-effective and also up to date to expose me to the material. I needed something comprehensive. That's kind of why I chose the program."
@3:59 - Justin Scott Brathwaite: "The content was really good. It was just that sometimes the narration was a bit rushed."
@7:13 - Justin Scott Brathwaite: "I would consider revisiting the course. I think I would do a refresher. I'm thinking about doing the PM one because I just saw there was a project manager course."
@11:00 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "It gave you a little bit of background on the site side of things, I think."
@14:52 - Justin Scott Brathwaite: "The regulatory, ICH, GCP, SIV sections were the most relevant because I deal with a lot of regulatory in this position so it's kind of helped me to leverage that to work with sites."
@16:13 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "And what are some reasons that you would recommend this course to others based on your experience?"
@16:28 - Justin Scott Brathwaite: "I think it's a pretty comprehensive course. The content is there. I think it's especially good for people on a budget."
@17:37 - Justin Scott Brathwaite: "No problem. Thank you, Courtney. Thanks for having me."
CCRPS Reviews: Unber Mahmood From A Busy Mom & Foreign Medical Doctor to A Clinical Research Coordinator
Unber Mahmood's case study interview about her experience with the Clinical Research Coordinator (CRC) certification program February 2024 Graduate
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Unber Mahmood's case study reflecting a highly positive experience with the CCRPS's Clinical Research Coordinator program, which not only met her educational needs but also supported her professional growth in the clinical research field. Her feedback provides valuable insights that can help in refining the program further, particularly in enhancing the visual and interactive components of the course.
Key Takeaways
● Unber particularly appreciated the self-paced aspect of the course, which allowed her to balance her responsibilities as a mother and a professional without feeling overwhelmed.
● The course provided all the necessary basics and details needed for a thorough understanding of clinical research, comparable to on-the-job training.
● The availability of instructors for queries and the interactive nature of the platform were crucial in assisting her whenever she encountered difficulties, contributing to a positive learning experience.
● Her motivation to enroll stemmed from a need for a flexible, affordable, and detailed-oriented course that could provide her with the specific knowledge required for a career in clinical research.
Career Impact and Advancement:
● Completing the course significantly boosted Unber's confidence, particularly in applying for jobs where her new credentials were recognized. She noted an immediate impact on her career, with advancements in her professional role within the clinical research field following the course.
Support System:
● The availability of faculty for consultation through email was highlighted as a critical support feature, aiding her whenever she faced difficulties understanding the course material.
Motivation for Enrollment:
● Unber's decision to enroll was driven by her interest in clinical research and the need for a manageable course that fit her busy schedule and financial constraints. The program’s flexibility, cost-effectiveness, and the depth of its curriculum were decisive factors in her choice.
Positive Experience with Course Format:
● Unber emphasized the self-paced nature of the course as her favorite aspect, which significantly reduced her stress by allowing her to manage her learning schedule alongside her responsibilities as a mother and a medical professional.
Course Content and Accessibility:
● Unber appreciated the comprehensive and detailed content that did not skip essential parts despite the course's short duration. The platform offered a variety of courses, making it a one-stop resource for various certifications related to clinical research.
@0:32 Courtney Fulkerson: "How are you? Good. So thank you so much for tuning into this call and first of all, congrats on completing this CCRPS program. We're pretty much just going to take some time to go through some questions and kind of hear your feedback on the program and things that you liked about it, things you didn’t and kind of help us tailor the program from here on now. So once you're ready."
@1:00 Unber Mahmood: "Experience. So when I started this course, the most important and the most my favorite thing is that the course was self-paced."
@1:11 Unber Mahmood: "Yes, that was the best part because as a mom and as already working in the job, when I started this course, I did not feel any kind of pressure doing that course that I had to meet the deadlines or I had to do my questionnaires or I actually enjoyed the course only because it was not giving me any kind of pressure. It was self-paced. It was it gives me a time to read it properly and so this was the main part I really enjoyed doing it."
@1:48 Courtney Fulkerson: "Yeah, we get a lot of good feedback on the self-paced portion of it so it definitely helps."
@1:55 Unber Mahmood: "I was in a program where you're looking for when you chose CCRPS and where you pleased the content once you got started. Actually, when I started, I was already working in over-hospital, actually I'm a foreign medical doctor, and I'm already working in a Fairfax hospital. But I always have an interest in clinical research, and because when I was in my university also, I participated in one of the researches with my senior doctors."
@4:00 Courtney Fulkerson: "And looking back, is there any aspect of the course that you feel could have been enhanced to make the learning experience even more rewarding?"
@4:12 Unber Mahmood: "Actually, at some point, I didn't think that it should be, but most of the part was okay, but I think if there was more of a, you know, the drawings part, you can say?"
@4:30 Unber Mahmood: "Because for me, I don't know for other people, like for me, if I'm reading something and it's with the drawings and it's with the charts or it's with the, it becomes more interesting to read to just, to just like prevent that, oh, it's boring just to read the same, just to read the lines. But overall, but it, I don't feel like more often, but yeah, that you could enhance, but if you don't do it also, it's okay."
@6:05 Courtney Fulkerson: "It was the actually the whole website to navigate is very easy."
@6:10 Unber Mahmood: "Like, this is the actually that's the first step. Anybody could actually get attracted to CCRPS because the website itself is very easy to understand. As soon as you open the website, even still, I think I’ve done my course like a little bit in the past. But even still, if I wanted to repeat something, if I wanted to remind something in my in my course, I just sign in, I just go back to my course and just read it again, you know, just to make it understand. So the website itself is very easy. It's just you just see it on the first front page too. It's very easy to navigate to sign in to make your username, password and everything. So it's very easy."
@7:39 Courtney Fulkerson: "Would you ever consider revisiting the course for a refresher? And is there anything specific you want to see within a refresher course?"
@7:47 Unber Mahmood: "In the, um, the refresher course, just keep it self-based."
CCRPS Reviews: Transitioning from Physical Therapy to Clinical Research, Celina Moon Gained Crucial Insights & Confidence
Celina Moon took the Clinical Research Coordinator (CRC) certification course provided by CCRPS, with a focus on ICH GCP to support her career transition into clinical research.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Celina Moon's feedback provides valuable insights into the strengths and areas for improvement within the ICH GCP course. Her suggestions for more interactive and engaging content delivery, along with the inclusion of practical examples, could enhance the learning experience for future participants, especially those new to the field. Her positive assessment and the constructive criticism will be instrumental in refining the course to better meet learner needs and expectations.A graduate case study interview from Celina, a recent graduate of our ICH GCP course program.
Key Takeaways
● Celina found visual aids such as graphs, tables, and bullet points extremely helpful for her learning style, emphasizing the importance of visual elements in grasping complex information.
● She appreciated the ability to download course materials for future reference, suggesting it added significant value to her learning experience. However, she recommended including real-world documents like informed consent forms to better anchor the theoretical knowledge within practical contexts.
● The course notably boosted Celina’s confidence, reaffirming her decision to transition into clinical research. She found the adverse event/safety reporting section particularly valuable, highlighting the ethical treatment of human subjects, which resonated with her professional values and goals.
Visual Learning Tools:
● Celina highlighted the effectiveness of visual aids like graphs, tables, and bullet points, which helped her understand complex information more easily.
Interactive Features:
● The right-hand community feedback section was particularly useful, providing a platform for previewing key points and reinforcing learning, which Celina found beneficial for understanding and organizing the course material in her mind.
Content Depth and Relevance:
● Celina sought a comprehensive ICH GCP course to aid her career transition and found the course to be detailed and informative, adequately covering the necessary aspects of clinical research.
Favorite Moments:
● Celina appreciated the course's use of visual aids and the right-hand community feedback section, which helped preview and reinforce key points, aiding in her understanding and retention of course material.
Course Content:
● She was looking for an ICH GCP course to support her career change and found the course's depth and detail both adequate and informative.
Impact on Professional Confidence:
● Completing the course significantly boosted Celina's confidence in her ability to transition into and succeed in the field of clinical research. The course reassured her about her career change and enhanced her understanding of the ethical treatment of human subjects, which was crucial for her professional identity and values.
@2:15 - Celina Moon: "Yes. So what I really actually enjoyed is the parts on the slide that were set up in graphs and tables and bullet points. It just makes a lot more sense to my brain."
@4:39 - Courtney Fulkerson: "That's actually the first time I've heard such good feedback about that part."
@3:17 - Celina Moon: "And I also really liked that on the right-hand side... there was an opportunity where as a learner, I could have put my feedback and some other students kind of did a similar thing, kind of like a cliff notes version."
@3:57 - Courtney Fulkerson: "Those are a couple of features that I thought were really, really neat."
@4:07 - Celina Moon: "The way my brain works is it's very much how the pieces are sitting into the puzzle... And then as I heard all these details, I knew what part of the puzzle was fitting into with that."
@10:06 - Celina Moon: "Yes. So I am very passionate and working very hard for a career pivot... My mom has worked in the field for 25 years and she was one that recommended this course."
@5:59 - Courtney Fulkerson: "And so you got in, you liked the content and just you felt like it was adequate to what you were trying to learn?"
@6:32 - Celina Moon: "It was more. It wasn't more than I expected, but... it helped me gain a better grasp of what I'm getting into."
@31:25 - Celina Moon: "I would definitely revisit it. Like I said, as I already found it, like I was excited, I downloaded the information."
@16:33 - Courtney Fulkerson: "So I'm glad you had a good experience."
@30:24 - Celina Moon: "Oh, I would definitely choose to take it again."
@22:25 - Celina Moon: "Yes, it actually did boost my confidence because at first... And then, you know, I took a deep breath and I started over and, you know, maximum attention, I was like, no, I do get it."
@33:42 - Courtney Fulkerson: "And any input is good input, like I said earlier, so it will help us just refine the course for the future and just meet your graphic. I really appreciate you and I wish you the best of luck."
@33:46 - Celina Moon: "Oh, thank you. I appreciate you too."
CCRPS Reviews: From Clinical Research Novice to Certified Expert: Stephanie's ICHGCP Course Journey
Stephanie completed the ICH GCP certification course offered by CCRPS, aiming to build a solid foundation in Good Clinical Practice as she pursued a career in clinical research.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Stephanie’s feedback underscores the effectiveness of the ICH GCP course in providing a solid educational foundation in Good Clinical Practice. Her suggestions for technical improvements point to areas where the course could be enhanced to ensure clarity and engagement for future participants. Overall, her positive experience and the tangible impact on her career trajectory demonstrate the value of the course in preparing graduates for professional roles in clinical research.
Key Takeaways
● Stephanie found the course content comprehensive, providing a good foundational knowledge of ICH GCP, which is crucial for anyone entering clinical research. The video format was highlighted as particularly effective for following along and understanding complex topics.
● The end-of-video exercises were highly beneficial for applying theoretical knowledge to practical situations, enhancing the learning experience by tying concepts to real-world clinical research scenarios.
● Completing the course significantly boosted Stephanie’s confidence, particularly in job interviews, and was instrumental in her securing a role as a research assistant. She specifically found the informed consent section thoroughly beneficial, which directly contributed to her professional capabilities.
End-of-Video Exercises:
● The exercises provided at the end of each video segment were highlighted as a crucial learning tool. These activities helped Stephanie apply the theoretical knowledge she gained in practical, clinical research settings, reinforcing her understanding.
Technical Accessibility:
● She appreciated that the course did not require affiliation with any specific company, making it accessible to a broader range of students, including those like her who were not connected to a corporate entity. This inclusivity is crucial for independent students seeking certification.
Confidence and Career Impact:
● Completing the course significantly boosted Stephanie’s confidence, especially during job interviews. The certification enhanced her resume, giving her an edge in the competitive research field and helping her secure a research assistant position.
Video Format:
● The use of video as the primary medium for content delivery was particularly effective for Stephanie. She noted that it was easier to engage with than traditional text-based materials or slides, enhancing her learning experience.
Overall Impressions:
● Stephanie appreciated the structured breakdown of the course into distinct topics and categories, which made the content accessible and easy to digest.
Course Format and Accessibility:
● The course’s pacing and structure (divided into 30-60 minute video segments) were well-received, making it manageable within a busy schedule.
Impact on Professional Development:
● The certification added significant value to Stephanie’s resume, enhancing her credibility in the field of clinical research and aiding in her career advancement.
@0:00 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "This meeting is being recorded. Thanks for being here today and sharing your experience with the program. Congrats on finishing it!"
@1:00 - Stephanie: "We didn’t have a course specifically for ICHGCP, and it was really good to get an introduction to how it works and procedures. I liked that it was in video form, which was easier to follow along than just slides or articles."
@1:52 - Stephanie: "At the end, they had those little exercises that you could do. It helped me apply the knowledge that I was learning in a more practical sense and get a sense of how this could be used in the clinical setting."
@2:25 - Stephanie: "I was looking specifically for ICHGCP and something that could help me get certified. I felt that it covered every aspect of GCP and was up to date."
@8:30 - Stephanie: "I added it as a certification in my resume, and I feel it enhanced my profile. It helped make me more credible as someone going into the clinical research field."
@9:17 - Stephanie: "I found the platform was very easy to follow. It was straightforward, and everything was in order, which helps you know exactly where to go and what to do."
@10:14 - Courtney Fulkerson: "What part of the course did you find most engaging and beneficial to your professional development?"
@10:24 - Stephanie: "I really liked learning about informed consent because they went into a lot more detail than I expected."
@11:10 - Stephanie: "I would recommend it because it's self-paced, accessible, and the content is straightforward. It's really good for beginners or even just people who are trying to refresh their memory."
@12:57 - Stephanie: "Thank you. It was really nice speaking to you."
@12:59 - Stephanie: "Thank you. Bye-bye."
CCRPS Reviews: Marta Marszalek's Pathway to Enhanced Professional Credibility
Marta Marszalek completed the CCRPS certification course, seeking to deepen her theoretical knowledge and practical skills in clinical research to complement her monitoring activities and comply with industry standards.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Marta Marszalek's feedback underscores the strengths of the CCRPS certification course in the self-paced learning and its adaptability to individual needs. Her constructive suggestions for enhancing lecture delivery and incorporating practical examples provide actionable insights for course developers. Overall, her positive experience and the tangible benefits she derived in terms of career advancement highlight the course's effectiveness in equipping professionals with the necessary skills for clinical research roles.
Key Takeaways
● Marta appreciated the self-paced nature of the course, which allowed her to organize her learning schedule to fit her personal and professional commitments.
● The course significantly boosted Marta’s confidence and prepared her for career advancement, leading to more job offers and enhanced professional credibility.
● The minimalist and visually engaging platform was noted for facilitating a smooth learning process, aligning well with her preference for a straightforward and distraction-free interface.
End-of-Video Exercises:
● The exercises provided at the end of each video segment were highlighted as a crucial learning tool. These activities helped Stephanie apply the theoretical knowledge she gained in practical, clinical research settings, reinforcing her understanding.
Interactive Elements:
● She enjoyed the interactive elements of the course, like the end-of-video exercises. However, Marta suggested that these could be expanded to include more frequent quizzes or interactive scenarios that reinforce the learning material throughout the course.
Impact on Professional Development:
● Adding the certification to her CV and LinkedIn profile significantly increased her marketability and helped secure new job opportunities, affirming the practical benefits of the course.
View the transcript of our case study here.
Overall Impressions:
● Marta found the course well-structured and accessible, particularly valuing the ability to learn independently while having access to external support.
Learning Enhancements:
● Marta appreciated the self-assessment opportunities, which helped identify areas that needed review, although she highlighted the monotonous delivery of some lectures as a point for improvement.
Course Format and Accessibility:
● Open to contributing as a course advisor, Marta is willing to provide recommendations to enhance future editions of the course, reflecting her vested interest in the continuous improvement of the curriculum.
Impact on Professional Development:
● Adding the certification to her CV and LinkedIn profile significantly increased her marketability and helped secure new job opportunities, affirming the practical benefits of the course.
@3:24 - Marta Marszalek: "Probably when I finished, when I started the test, and it was all positive. But actually, I also like the moments when I was not passing this self-evaluation test. And then I knew which part I have to go back, which part I have to go back and learn again. But actually, now I have to say that the most exciting was the beginning, because you know, you are starting with something new."
@4:22 - Marta Marszalek: "Well, it was actually exactly what I was looking for. So because I wanted to find the course that I could learn by myself, but with some external help. So for me, it was important to see the presentations and to find the speakers going through the presentations. So that was what I was looking for. And of course, the certificate gates were important."
@5:00 - Marta Marszalek: "The learning experience, even more rewarding? Like one thing I would consider as a possibility to improvement would be like the lectures but the lectures that were reading the slides. So first it would be better if they just don’t read everything."
@6:26 - Marta Marszalek: "No, I think it was covered pretty much well with theoretical parts. Maybe some like cases would be nice to include. I always like to see with the tests or like evaluations. So you can see like the case and you can evaluate what’s what’s wrong with this one, where’s the mistake or something like that."
@7:26 - Marta Marszalek: "Yes, I think the courses is prepared very well. It’s not very expensive, so it’s very good. I think it can be even more expensive if you can offer more things because people So I think with some improvements I think it could be more expensive but also so, um, the marketing of your, uh, the course is like, it’s, um, it’s not very well, uh, shown. Like, yeah, I think you can show more from the, uh, what you can get from the course."
@8:33 - Marta Marszalek: "If I want to do a refresher course. Yeah. Yeah, definitely."
@10:36 - Marta Marszalek: "So in my case, I needed the certificates to obtain the certificate in very fast, in very fast way. so lots of courses, they would tell you how many hours you can do per week or they are already organized. But in this case, I knew that I would sit. a few days, 10 hours straight, and I can do it. for me, with the flexibility to organize your learning time and schedule, that was very important and the easiness to do this course."
@11:57 - Marta Marszalek: "So I was already working in clinical training. class when I, in the first one, that was, I was not doing, like, strictly monitoring activities, but I was collaborating also for monitoring visits, and for the compliance reasons, I needed the certificate, and I wanted to learn more because I knew the practical parts, and some theory as well, but it’s different when you have to refresh everything and you have to do, it’s like master and with any kind of studies that we’re doing, so in my case it was necessary. I can say I’m not sure if this is because of the certificate or different reasons, but I do receive a lot of offers, job offers, including, for example, yeah, and I’m sure it’s a great value to add to your CV."
@14:00 - Marta Marszalek: "Definitely very easy. That was also one of the reasons that I chose because I was playing with platforms and I’m very like visual person so I need to see that it was simple. It was not like very distracting. I don’t like it but to my images colors and it was very easy. I’m a minimalist person so it’s easy to open it to get there and it was also nice that when you were entering the platform it was you were going to when you left it."
@15:13 - Marta Marszalek: "Basically, what I just said, I think it’s very good for people that they are self-discipline. They like learning new things or they like refreshing things. I think people who are also like challenges, so that’s why you are doing courses when you get some certificate games, it’s always like... Engaging you more in learning something new. Okay. Yeah."
CCRPS Reviews: Katie Decker, From Clinical Research Receptionist to Certified Study Coordinator
Katie Decker completed the CCRPS certification course, aiming to bolster her extensive practical experience in clinical research with formal training. This certification enhanced her professional profile by validating her knowledge and skills, aligning with industry standards, and preparing her for advanced roles in clinical research.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Katie Decker's feedback from the CCRPS certification program highlights the effectiveness of the course in reinforcing and expanding her knowledge in clinical research. Her suggestions for incorporating more interactive elements and enhancing the marketing of the course's flexibility are valuable insights that could help improve the learning experience for future participants. Overall, her positive review and the tangible benefits she has experienced in her career underscore the value of the CCRPS program in professional development.
Key Takeaways
● Katie appreciated the self-paced online format, which allowed her to manage her learning alongside work commitments efficiently.
● The certification significantly boosted Katie's confidence and enhanced her resume, making it more compelling to potential employers.
Professional Background and Progression:
● With seven years of experience in research roles, progressing from receptionist to study coordinator, Katie has learned significantly on the job. The certification added formal acknowledgment of her skills and knowledge, leading to increased job offers and opportunities.
Learning Platform Experience:
● The platform was easy to navigate and allowed Katie to pick up where she left off, which she found convenient for integrating learning into her busy schedule.
Confidence and Career Advancement:
●The certification has not only enhanced Katie's resume but also her confidence, particularly evident during job interviews and professional interactions.
Course Experience and Content:
● Katie enjoyed the accomplishment of completing the course and obtaining her certification. She valued the course as a good refresher on research basics and appreciated learning new aspects of clinical research.
Motivation and Outcomes:
● Choosing the course for its self-paced format, Katie needed a certification that could be completed alongside her job without requiring a degree. The course fulfilled these requirements and prepared her for career advancement.
Engaging and Beneficial Course Aspects:
● Taking exams and realizing the depth of her knowledge was particularly satisfying for Katie, reaffirming her competence in her field.
@0:00 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "This meeting is being recorded. Sorry, let me make sure everything. Okay. I think we're good to go. All right. Well, first of all, thank you so much for meeting with me and congrats on completing the CCRPS program."
@0:41 - Katie Decker: "Definitely completing the course, obviously. I worked in research for like seven years now. So it was kind of cool to have like a refresher on the basics and also kind of learning a couple new things here and there. But definitely just completing the course is very accomplishing."
@1:04 - Katie Decker: "Um, so I actually my employer signed me up for the class and everything. Um, originally I worked for a different employer and they were going to have me... I don't know the name of the program, but it was where I actually had to sit for an in-person exam... Um, so this wasn't what I was expecting when I first started the classes and I loved that it was online and kind of self-paced. So, um, it was super like user friendly and very easy and convenient with my work schedule to do."
@3:54 - Katie Decker: "Yeah, definitely. It was nice because I could do it at work and like in between patients kind of hop on and do a couple pages."
@4:10 - Katie Decker: "Definitely. I think for a refresher course, it's always good to kind of refresh on documenting AEs and SAEs and the informed consent process."
@8:19 - Katie Decker: "So I did add the certification on to my personal resume, and then I have a LinkedIn profile and also Indeed. So I have like a picture of like the actual certification with a little serial number and everything on there."
@8:44 - Katie Decker: "And yeah, definitely. And it's nice to when you can, like, if your computer locks or whatever, when you log in, it just brings you right back to where you are, which was really convenient."
@9:00 - Katie Decker: "since ankle and a core research. I just think overall it's just nice to have any kind of extra certification or like a document under your belt... So like for me, like they just called me a coordinator, which I know it's still the same thing. But now I have like the ACRC like my signature and all my name tag, which is nice. So it kind of, you know, always a confidence boost."
@9:32 - Katie Decker: "And this might sound weird, but I guess kind of just taking the exam was kind of just nice like, Oh, I know that. It's easy. the answer to that's so easy. So it kind of just makes me like realize what, wow, I actually do kind of know more of what I'm doing."
@11:03 - Katie Decker: "I think it kind of helps me like be more motivated to go back to school. Eventually, I do want to be a CRA. So I think it's just kind of nice just with already being coordinated for so long. Just having that kind of like title now, like credit it, kind of versus coordinator. It kind of just helps boost my career. It kind of like just makes my resume a little more beefy or two and helps boost my knowledge."
@11:44 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS): "Thank you. I'll send you an email or go get email with your gift card for doing this with me and it should just come through email. But if you have any problems, just definitely reach out to me."
@11:58 - Katie Decker: "Have a good day. You too."
CCRPS Reviews: Aishwarya Sukumar, From International CRC to U.S. Lead CRC and CRA
Aishwarya Sukumar completed the CCRPS certification course to enhance her understanding of FDA regulations and adapt to the clinical trials industry in the United States after relocating from abroad. This certification broadened her professional scope, solidified her theoretical foundations, and equipped her for higher positions within clinical research, transitioning from a Clinical Research Coordinator to a Clinical Research Associate role.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Aishwarya Sukumar's feedback underscoring the CCRPS certification program's effectiveness in accommodating diverse learning needs while suggesting areas for enhancement such as increased practical engagement and better support systems. Her progression in her career illustrates the tangible benefits of the course, advocating for its value in professional development within clinical research.
Key Takeaways
● Aishwarya appreciated the self-paced online format which provided the flexibility needed to balance her studies with personal responsibilities.
● The course significantly boosted her confidence, enhanced her resume, and equipped her with the knowledge necessary to progress in her career from a CRC role to a CRA position.
Learning Platform Usability:
● Found the platform easy to navigate and appreciated being able to return to the last point of study seamlessly, which suited her intermittent learning sessions.
Career Advancement:
● Successfully transitioned from a CRC to a Lead CRC, and then to a CRA role, underscoring the significant career advancement facilitated by the course.
Engaging Learning Components:
● The most engaging aspect was the ability to revisit previous modules to reinforce learning, which she found crucial for mastering complex topics.
Course Format and Learning Experience:
● The self-paced nature and extended timeline allowed Aishwarya to effectively manage her study schedule without compromising her responsibilities as a new mother.
Motivation for Course Selection:
● Chose the CCRPS program for its affordability and flexibility, crucial as she was adjusting to new motherhood and transitioning careers in a new country.
Professional Growth:
● The course facilitated a smooth transition into the clinical research field in the U.S., providing her with necessary certifications and boosting her credibility in job interviews.
@0:03 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"This meeting is being recorded."
@2:36 - Aishwarya’s iPhone:
"Sure, please go ahead."
@2:38 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"Could you share some of your favorite moments from the course that enriched your learning experience?"
@2:45 - Aishwarya’s iPhone:
"So the one thing which I would do is the self-paced thing, which is like much easier for homemakers but to choose my own time and I get to study when I... like my energy is full on so I can go ahead and study and focus more so that’s a good part and the other thing is also they though they give you a time frame of three months you can still go ahead and refresh back to your previous texts or other previous chapters in case you have any questions there you can go ahead and check back and then look back to the newest chapter if you can correlate between those two things yeah so that one was an amazing feature and you may take enough time so they say even those three months you can go up to four months and take your own time and understanding each concepts yeah that was a good part there yeah and I feel like you can just make it fit your schedule and your learning style right yeah right..."
@5:09 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"Okay, good. And looking back, is there any aspect of the course that you feel could have been enhanced to make the learning experience more rewarding for you?"
@5:23 - Aishwarya’s iPhone:
"Yes, I did feel that at one point because I was stuck with a question because I’ve been going through the programs and I was towards the end of the program."
@6:25 - Aishwarya’s iPhone:
"But in a way that I would say that if this could be a support for job or just like even reaching out to a contacts or somebody who can lead on what path you can take next, that would be a great benefit to the program."
@7:53 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"So no complaints there And when you were considering enrolling was there any specific information? information on our website that could have better highlighted the benefits of the course?"
@10:11 - Aishwarya’s iPhone:
"So, okay. Yeah, I mean, it’s been a while since I’ve been in the course. have to provide that."
@12:37 - Aishwarya’s iPhone:
"So that is always great to hear. Right."
@14:07 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"Yeah, that’s, that’s always good. And how long did it take you to complete the course?"
@15:09 - Aishwarya’s iPhone:
"So I started updating my resume with all the information which I learned and I also put my certification as in here because I mean I did receive the certification so I did that."
@15:33 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"And how would you describe your experience with our learning platform? Did it facilitate a smooth and engaging learning process?"
@16:12 - Aishwarya’s iPhone:
"Yeah, in terms of boosting my confidence because I was like in the New Year in the headlights, I didn’t know many things when I started in here and when I learned the course, kind of learned the new terminologies are the acronyms I would say."
@18:57 - Courtney Fulkerson (Certified Clinical Research Professionals Society CCRPS):
"And now I’m a CRA as well there. It’s awesome, yeah, so I feel like your career definitely has advanced since you completed the course and Apply what you learned from that into Yeah, I mean, yeah, if time permits everyone that one then I can go ahead and check back like just to refresh my memory Yes, well that completes our interview.
CCRPS Reviews: Renata Noronha's Journey: Learning to Leadership in Clinical Research
Renata Noronha completed the CCRPS certification to transition from pharmacovigilance to clinical research. This certification enriched her understanding of international PV requirements and helped her secure a job at a clinical research organization, advancing her from an associate to a lead safety associate.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Renata Noronha’s feedback providing valuable insights into the strengths and areas for improvement of the CCRPS certification program. Her positive experience and constructive suggestions will be instrumental in enhancing the course structure, content, and delivery, ultimately enriching the learning experience for future participants aiming to advance their careers in clinical research.
Key Takeaways
● Renata appreciated the clear, structured format of the course, highlighting its ease of follow-through which enhanced her learning experience.
● Enjoyed the global perspective on pharmacovigilance (PV) requirements which added valuable insights applicable to her career in clinical research.
● The certification was pivotal in securing her new role at a clinical research organization, aiding her transition from an associate to a lead safety associate.
● Benefited from the self-paced nature of the course, which allowed her to expedite her learning to align with career opportunities.
Impact on Career:
● The course significantly boosted her confidence in job interviews, equipped with new knowledge and a credible certification.
● Directly contributed to her rapid career progression within her current organization.
View the transcript of our case study here.
Learning Experience:
● Found the course modules well-organized and straightforward, aiding her comprehension and retention of information.
Motivation and Background:
● Chose the CCRPS program to facilitate a career shift from pharmacovigilance to clinical research, driven by the course’s specialized focus and comprehensive coverage of necessary skills and knowledge.
@0:59 Courtney Fulkerson: "Okay. Perfect. Great. Could you share some of your favorite moments from the course that enriched your learning experience?"
@1:05 Renata: "Okay, so I think I found that like easy to follow. I liked it was structured. It was easy to follow. And um, I had previous experience in pharmacovigilance. So it was a bit hard for me to understand Like where like what things are there were a few things that were new to me, especially the reporting with uh, different countries."
@2:33 Renata: "So, I was recently let go from my company. They weren't doing well during COVID, and so it was a natural whole product company, so I did pharmacovigilance for them. When I was looking at jobs in my field, clinical trials were one of them. So, I wanted to get more experience regarding pharmacovigilance first, and then I found this program through one of my friends in clinical research."
@2:33 Courtney Fulkerson: "That sounds like it was a really good fit for what you were looking for. It's great to hear it worked out pretty well for you."
@10:16 Renata: "It is certification. yeah. That’s everything."
@10:18 Courtney Fulkerson: "Okay, so that's good. It sounds like the certification was a significant achievement for you."
@11:04 Renata: "Yeah, I think, like, I think I would."
@12:05 Renata: "So I know that what stood out for me was first, it was clinical research and it was a field that I was interested in getting into, it's specialized. That's important to me."
@12:38 Courtney Fulkerson: "Right, it's always crucial to find a course that not only provides knowledge but also aligns with your career goals."
@15:13 Renata: "Yeah, I had to fast track myself because I got a job, so I had to really buckle down. I helped to have a deadline. So I think I started in November, October and I finished in December. I didn't have a Christmas break, I had to finish the course because my interview was at the end of December."
@16:00 Courtney Fulkerson: "That's quite a commitment! It's impressive that you managed to complete it so quickly."
@16:27 Renata: "Yes, so it's good. I finished it because once I started my job, all the clients we have sponsors they all want your resume. Having that on your resume really helps develop rapport with the sponsors."
@21:29 Renata: "Since I've completed the course, I got a job at a clinical research organization."
@21:35 Courtney Fulkerson: "So that's great. It sounds like the course really helped bridge the gap to your new role."
CCRP Reviews: Dr. Vrushali Borawake's Journey: From Certification to Clinical Research Success
Dr. Vrushali Borawake transitioned from medicine in India to clinical research in Germany after completing the CCRPS certification. This program enhanced her job interview confidence and provided her with key industry knowledge, enabling her to secure roles as a CRC, CRA, and now a project manager. Dr. Borawake recommends this course to international medical professionals seeking careers in clinical research.
In preparation for further accreditation for CCRPS, we are conducting case studies with graduates to discuss their experience in our course in 15-30 minute interviews with our clinical research educator, Courtney. We invited students to share their feedback asking extensive questions on why they chose the course, what they felt while taking the course, and how the course has helped them after completion. Today, we share Dr. Vrushali Borawake's feedback underscores the CCRPS program's significant impact on her career transition and advancement. Her suggestions for incorporating more interactive learning tools and her endorsement of the program's applicability across different regulatory environments provide valuable insights for future course enhancements. These improvements could further enrich the program's effectiveness in preparing professionals for successful careers in clinical research.
Key Takeaways
● Dr. Borawake successfully transitioned from medicine in India to clinical research in Germany, attributing significant career advancements to the CCRPS courses.
● She secured positions as a Clinical Research Coordinator (CRC) and Clinical Research Associate (CRA), and has recently advanced to a Project Manager role.
● The courses provided her with essential knowledge and confidence, particularly in job interviews, by familiarizing her with industry-specific terminology and documentation.
● Dr. Borawake highlighted the course's effectiveness for international medical professionals seeking to enter the clinical research field in Germany, noting its comprehensive coverage of relevant regulations and practical applications.
Professional Growth:
● Credits the CCRPS program with a rapid progression in her career, moving from CRC to CRA, and now to a project management role within a significantly shortened timeline compared to industry norms.
Recommendations to Others:
● Strongly recommends the course to other international medical professionals in Germany, emphasizing its relevance and adaptability to European/German regulations.
View the transcript of our case study here.
Learning Experience:
● Appreciated the structured approach that covered clinical research from start to finish, which was instrumental in her understanding of the field.
● Found the mix of PDFs, videos, and real-document examples particularly beneficial in preparing for real-world applications.
Application of Knowledge:
● The course content directly influenced her ability to perform effectively in her roles, especially with tasks like site suitability forms and other CRC/CRA responsibilities.
@0:01 Courtney Fulkerson: "Okay. Okay. All right. Well, like I said, we're just going to go through some questions. So whenever you're ready, we're going to get started."
@0:13 Vrushali Borawake: "Yeah."
@0:18 Vrushali Borawake: "Sure, basically it has helped me so much that I've got two courses. So initially when I shifted from India, it was, I was a medical practitioner of an alternative medicine. And then when I shifted to Germany, I was getting calls for a partner job, but not for an associate CRA job. So I did the first course for CRA and then a second course for project management and clinical research."
@0:47 Courtney Fulkerson: "That’s awesome. To come back and decide to do another program."
@0:52 Vrushali Borawake: "So that’s really good. Yeah. Definitely. So the first offer I got was from the first course as a CRA and that was an associate. So the second office they were saying we can offer a project manager position as well so that you can learn hands-on while working. And then I thought okay, I start on the post of me. So I thought before I start, I'll just buy again from CCRPS because this course has always helped. So right now I feel I’m fully prepared for the post of me."
@1:24 Courtney Fulkerson: "That’s awesome, yeah. Well hopefully it all works out and hopefully you’re able to use what you learned from the course into your job."
@1:33 Vrushali Borawake: "So that’s really good. Sure, sure, definitely. So coming to your question from the first course, what I liked about what I found very interesting about CRA course that it was from initially into end of the study and not just like considering that you’re already a coordinator and then you’re shifting to associate side. So the first few slides were differentiation between CRC and CRA and I’m not Because everybody knows that. So while working, there is a difference we monitor in coordinator, but it was very well structured."
@2:38 Courtney Fulkerson: "Good. And so you feel like you were able to kind of put that into practice really as soon as you got into the role."
@2:58 Vrushali Borawake: "Yeah, yeah, immediately. Yeah."
@3:01 Courtney Fulkerson: "Good. Okay. And could you share some of your favorite moments from the course that enrich your learning experience?"
@3:07 Vrushali Borawake: "Oh wait. That’s when I just asked you. so sorry."
@3:09 Courtney Fulkerson: "What sort of program are you looking for when you chose CCRPS and were you pleased with the content of the course once you got started?"
@3:18 Vrushali Borawake: "So I would like you to explain a bit to the question."
@3:23 Courtney Fulkerson: "So what kind of program, whenever you were looking to start a program, kind of what were you looking for and how did our program stand out amongst the other ones and kind of like what aspects were you looking to meet your certain criteria?"
@3:38 Vrushali Borawake: "Sure. when I was searching, I was searching for a coordinated course or something. But to be honest, I went, I always go into chat GPT, I went into chat GPT, but what are the best courses for to land a job as a CRE or CRC and CCRPS definitely comes into first fight. So you have the first two I saw were not online so I had to be in the United States to do that, that is not possible and I think the others I don’t remember but the most suitable was CCAT is because of the online schedule, immediate payment and I think it was also a very reasonable amount, it was around 500 euros, $5.20 or something like that and then I decided for that because of online it was self-paced so I could do at home, initially they had given a timeline that if you follow the schedule you can do it in one month or something but I had like seven days to start my job so I took care of this too, it was very interesting the things that I knew I could skip, that’s a good part but not entire course I could skip that is also good part for people who just want to have a certificate it makes us sure that you attend it and The quiz at the end was also good so I finished the course in three to four days but for the quiz I went through the documents once again just to be sure that I pass it. The best and the most important part was the certificate was generated very easily."